Elevated plasma glucose levels upon hospital admission, known as acute hyperglycemia, are prevalent among patients experiencing acute myocardial infarction (AMI), affecting up to 50% of cases, depending on the defined glycemic threshold and presence of diabetes [1,2,3,4]. Acute hyperglycemia has been recognized as a significant independent predictor of both in-hospital morbidity and mortality in patients with AMI, regardless of diabetes status [4, 5]. Notably, for every 18 mg/dL (1 mmol/L) rise in glucose levels above 200 mg/dL, there is an associated 5% increase in hospital mortality risk for patients with AMI with diabetes and a 4% increase for those without diabetes [6]. However, the adverse effects of glucose dysregulation extend beyond acute hyperglycemia to include fluctuations in glycemic values, with acute glucose changes in both directions [7]. Indeed, increasing evidence suggests that hypoglycemia and, more recently, glycemic variability (GV) in hospitalized patients with AMI are emerging concerns closely linked to prognosis, thus highlighting their importance as potential therapeutic targets. In AMI, acute fluctuations in glucose levels appear to have more detrimental effects on the development of major cardiovascular complications compared to acute hyperglycemia.
This review will delve into the clinical and prognostic significance of hypoglycemia and GV in patients with AMI, while also exploring current insights into the potential mechanisms underlying the association between these glucose dysregulations and adverse outcomes. Ultimately, we advocate for the incorporation of hypoglycemia prevention and GV reduction as standard treatment objectives during AMI management.
To address these objectives, relevant literature was identified through a comprehensive search of the PubMed, MEDLINE, and Scopus databases. The search was conducted using combinations of the following keywords: “acute myocardial infarction,” “hypoglycemia,” “glycemic variability,” “blood glucose fluctuations,” “prognosis,” “cardiovascular outcomes,” and “continuous glucose monitoring.” Additional studies were identified through manual searches of reference lists from key articles and systematic reviews. Included studies encompassed original clinical research, meta-analyses, and expert consensus statements published in English between January 2000 and May 2025. Priority was given to studies involving hospitalized adult patients with AMI. Studies focusing on glucose abnormalities outside the context of AMI or involving non-human models or performed before 2000 were excluded unless they contributed mechanistic insight relevant to the topic.
Hypoglycemia in AMI
Hypoglycemia is a clinical condition characterized by an abnormally low level of blood glucose [8]. Establishing a single plasma glucose level indicative of hypoglycemia is challenging, as the glycemic threshold for symptom development varies depending on the individual’s diabetes status and their chronic glycemic control [9, 10]. Nonetheless, hypoglycemia is typically defined as a blood glucose concentration below 70 mg/dL (3.9 mmol/L), with a critical alert value below 54 mg/dL (3.0 mmol/L), which can lead to severe neurological consequences, including potentially fatal outcomes, if not promptly addressed [11]. Hypoglycemia may manifest with or without typical adrenergic symptoms such as sweating, palpitations, trembling, and tingling. Additionally, hypoglycemia is most commonly induced iatrogenically as a result of anti-hyperglycemic therapy, particularly with insulin, or less commonly, it may occur spontaneously, possibly due to a more serious underlying disease [4, 12,13,14].
Incidence of hypoglycemia in AMI
Data from the Cooperative Cardiovascular Project, encompassing a substantial American community-based sample of 141,680 elderly patients hospitalized with AMI from 1994 to 1996, revealed that approximately 15% of patients had admission glucose values lower than 110 mg/dL. However, this frequency varied, ranging from 2% among patients without diabetes to 40% among those with diabetes [15]. In a subsequent analysis of a database comprising 23,613 patients with AMI admitted to 40 hospitals across the United States between 2000 and 2005, the incidence of critical hypoglycemia (below 60 mg/dL) during hospitalization was approximately 6%. One-third of hypoglycemic events occurred spontaneously, while the majority followed insulin administration [13]. Another study, involving a post hoc analysis of two large trials with 30,536 patients with AMI, reported admission hypoglycemia (below 70 mg/dL; < 3.9 mmol/L) in 1.5% of patients, with an additional 5% experiencing hypoglycemic events within the subsequent 24 h after admission [16]. More recently, among 34,943 patients with AMI from two registries, the Korea Acute Myocardial Infarction Registry (KAMIR) and the Korea Working Group on Myocardial Infarction (KorMI), 1.2% had admission glucose levels below 70 mg/dL (< 3.9 mmol/L) [4]. Hypoglycemia is even more prevalent among AMI patients with diabetes. In the Diabetes Mellitus Insulin Glucose Infusion in Acute Myocardial Infarction 2 (DIGAMI 2) trial, hypoglycemic episodes were observed in 12% of all patients with diabetes [17]. Furthermore, the likelihood of hypoglycemia increases to up to 8% when considering only patients with ST-elevation myocardial infarction (STEMI) [18].
Prognostic relevance of hypoglycemia in AMI
Similar to the well-established link between acute hyperglycemia and heightened morbidity and mortality in patients with AMI, the presence of hypoglycemia has been associated with a poorer prognosis. Numerous observational studies have demonstrated that hypoglycemia during hospitalization for AMI is closely correlated with increased mortality rates. In one of the earliest studies focusing on the clinical significance of glycemia in AMI, Pinto et al. [18] reported a U-shaped relationship between admission glucose levels and adverse outcomes. Specifically, the incidence of death or recurrent AMI within 30 days in STEMI patients was higher in cases of hypoglycemia (10.5%) or hyperglycemia (7.2%) compared to euglycemia (4.2%). A similar distribution of 30-day mortality rates based on admission glucose levels was observed by Lee et al. [4] in a large cohort of patients with AMI. This trend was evident across the entire population as well as among patients with and without diabetes analyzed separately. Notably, hypoglycemia was associated with an almost fivefold higher risk of mortality in patients without diabetes and an almost threefold higher risk in those with diabetes. This discrepancy may be attributed to the greater prognostic impact of spontaneous hypoglycemia, which is more common in patients without diabetes, compared to iatrogenic hypoglycemia. Indeed, a retrospective cohort study by Kosiborod et al. [19] revealed that in patients hospitalized with AMI, hypoglycemia was linked to increased mortality only in those experiencing spontaneous hypoglycemia (18% vs. 9% of patients not experiencing hypoglycemia). Conversely, iatrogenic hypoglycemia following insulin therapy was not associated with a higher mortality risk (10%). Consistent with this, an analysis from the DIGAMI 2 trial, which focused on the safety and efficacy of insulin use in hyperglycemic patients with diabetes during AMI, concluded that hypoglycemic episodes occurring within the first 24 h after admission do not elevate the risk of cardiovascular mortality, re-infarction, or stroke during hospitalization or in the subsequent three years.
The association between hypoglycemia during hospitalization for AMI and adverse outcomes has also been observed in long-term follow-up studies. Retrospective analyses of patients admitted for acute coronary syndromes revealed that hypoglycemia was an independent predictor of all-cause mortality up to three years post-hospitalization [20].
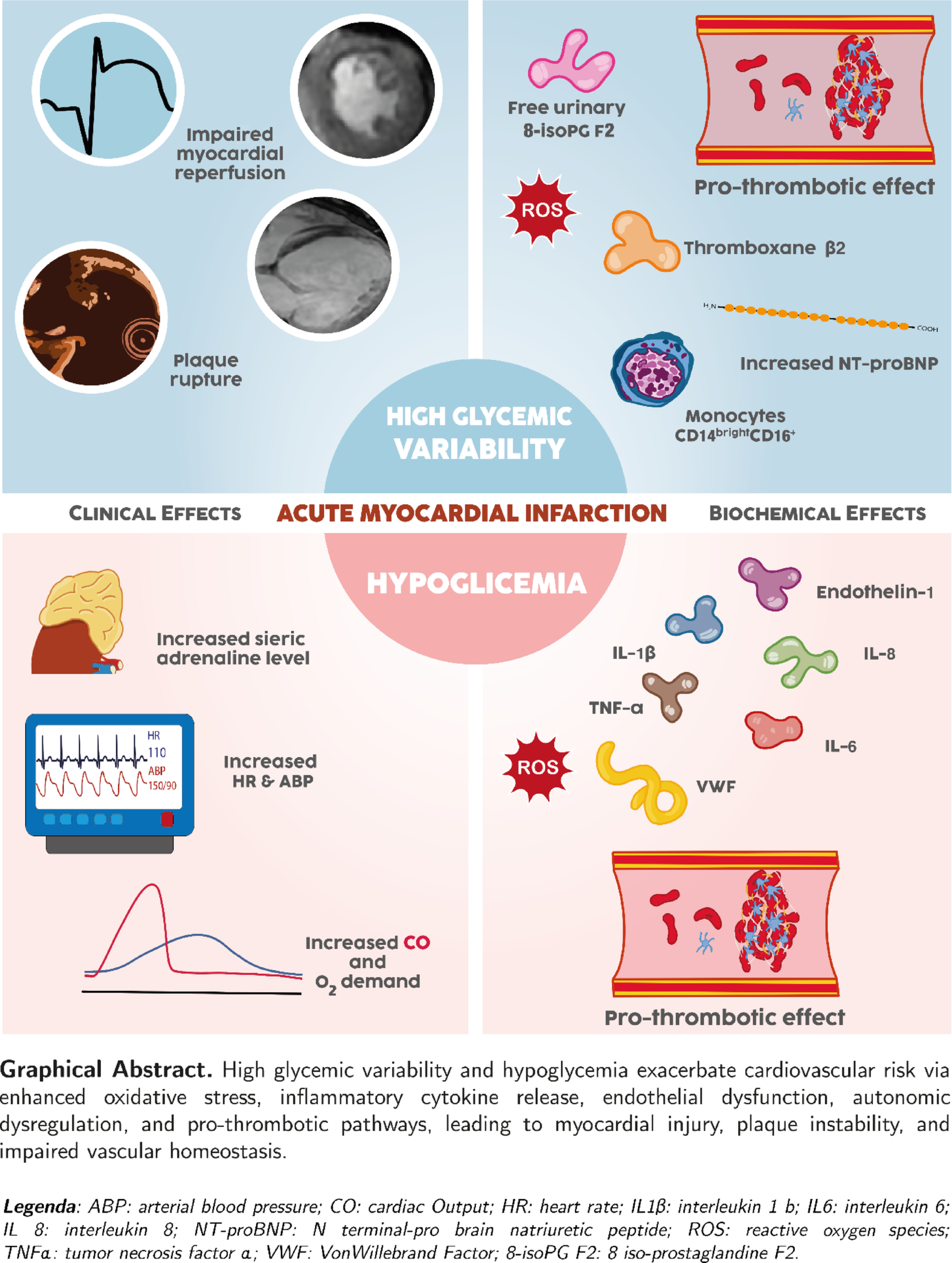
Possible mechanisms underlying the association between hypoglycemia and worse prognosis in AMI (Fig. 1). Hypoglycemia sets off a cascade of events that may elucidate its prognostic significance during hospitalization for AMI and in the aftermath of the acute event. The occurrence of hypoglycemia triggers an increase in plasma adrenaline levels, which subsequently elevate heart rate and systolic blood pressure, and an increase in left ventricular systolic function [21, 22], resulting in a 50%-100% rise in cardiac output [23, 24]. This substantial increase in cardiac workload and oxygen demand can be particularly detrimental in the context of acute myocardial ischemia. Additionally, acute hypoglycemia promotes endothelial dysfunction by increasing circulating levels of interleukins, cytokines, and reactive oxygen species. Notably, endothelin-1, a potent vasoconstrictor released by endothelial cells, has been shown to increase by up to 70% during hypoglycemia [25]. High-sensitivity C-reactive protein (hs-CRP) elevation during AMI is a widely observed finding. A prospective study of 2,064 patients showed that hs-CRP predicts in-hospital outcomes and two-year mortality in patients with AMI with and without diabetes [26]. Moreover, in patients with diabetes, the same risk of adverse events as in patients without diabetes is associated with higher hs-CRP levels. This suggests that the inflammation-related risk during AMI overlaps with the chronic inflammatory state associated with diabetes [26]. Also acute hypoglycemia may induce inflammation, as evidenced by significant elevations in hs-CRP levels and other proinflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, IL-6, and IL-8, following hypoglycemic episodes [27, 28].
Biochemical and clinical effects of hypoglycemia in acute myocardial infarction. ABP Arterial blood pressure, CK Cytokines, HR Heart rate, hs-CRP High sensitivity-C reactive protein, IL Interleukin, LVEF Left ventricular ejection fraction, NSVT Non-sustained ventricular tachycardia, ROS Reactive oxygen species, TNF Tumor necrosis factor, VW Von Willebrand
Numerous studies have demonstrated that acute hypoglycemia enhances platelet aggregation and clot activation, leading to a prothrombotic and procoagulant state [29, 30]. In patients with diabetes, acute hypoglycemia induces a significant increase in serum VIII factor levels [31]. Additionally, Von Willebrand factor is released from endothelial cells and platelets in response to low blood glucose, promoting platelet aggregation [32, 33]. Consequently, episodes of hypoglycemia appear to amplify many of the pathophysiological pathways already involved in acute myocardial ischemia, which are closely linked to prognosis [34].
Another mechanism through which hypoglycemia may impact outcomes in patients with AMI is its pro-arrhythmic effect. In patients hospitalized with STEMI undergoing primary percutaneous coronary intervention (PCI), hypoglycemia has been associated with a significantly higher frequency of ventricular arrhythmias, including ectopic ventricular beats and non-sustained ventricular tachycardias [35, 36]. Moreover, in various clinical contexts, hypoglycemia has been linked to an increased susceptibility to cardiac arrhythmias such as bradycardia, atrial fibrillation, and ventricular ectopic beats [37, 38]. Additionally, hypoglycemia has been implicated in QT interval prolongation, with a strong correlation observed between hypoglycemia, peak adrenaline blood levels, and QT lengthening, independent of extracellular potassium levels [38,39,40].
Finally, aside from a causal relationship between hypoglycemia and worse outcomes in AMI, hypoglycemia may also be considered an epiphenomenon of severe organ dysfunction, similarly as it has been proposed in septic shock [41]. The higher frequency of hypoglycemia during STEMI, the most severe form of AMI, and the association of adverse prognosis with spontaneous rather than iatrogenic hypoglycemia, suggest that low blood glucose levels may reflect greater severity of the ongoing acute event, identifying critically ill patients [4, 13, 14].
Glycemic variability in AMI
Glycemic variability is often overlooked in routine clinical practice but plays a crucial role in glycemic control. It encompasses fluctuations in blood glucose levels or related parameters of glucose regulation over a specific period. This concept includes two main types of measurements: short-term and long-term GV. Short-term variability encompasses fluctuations within a single day (within-day) as well as variations between different days (between-day), ranging from peak to trough levels. Long-term GV involves serial measurements over an extended period, including assessments of glycosylated hemoglobin and fasting and postprandial glucose levels [6, 42, 43].
Traditionally, self-monitoring of blood glucose has been the preferred method for evaluating GV [44]. However, continuous glucose monitoring (CGM) systems have become increasingly favored in recent years and are now considered the gold standard for assessing short-term GV [45, 46]. In the clinical context of AMI and other critical conditions, short-term GV is often defined using various metrics, leading to variability in its assessment. The most used metrics are the standard deviation (SD), defined as the variation over the mean glucose concentration, and the coefficient of variation (CV), defined as SD divided by the mean glucose concentration. Standard deviation is deeply related to the mean glucose level: CV should be preferred in patients who have higher mean glucose level, because they will have higher SD. Furthermore, CV is a metric relative to the mean, which makes it a better reflection of hypoglycemic fluctuations than SD alone. Another measure of within-day variability is the mean amplitude glycemic excursion (MAGE), which describes the average glycemia above one SD from the 24-h mean glycemia; the mean of daily differences (MODD) is considered to be the best metric of between-day GV and is obtained based on the differences between two glucose measurements at the same time within a 24-h period. Referring to GCM, continuous overall net glycemic action (CONGA) is another short-term GV metric assessed by the differences between glucose levels calculated ad regular time intervals and then on the SD of these differences [43]. The heterogeneity in reporting GV was largely due to the previous lack of standardized definitions, which was addressed by a consensus established in 2017 [46]. Therefore, more recent literature is expected to report GV more consistently, particularly when based on CGM data.
Incidence of high GV in AMI
In a retrospective study involving 18,563 patients hospitalized with AMI between 2000 and 2008, who underwent > 3 blood glucose determinations within the first 48 h, it was found that 50% of patients experienced a glucose level excursion of at least 64 mg/dL, with half of them having a glucose excursion exceeding 127 mg/dL [47]. In a more recent prospective study of 222 patients hospitalized with AMI, acute GV—defined as a MAGE > 70 mg/dL (> 3.9 mmol/L), detected through CGM systems within the first 48 h after admission—was observed in 36% of cases [48]. Similarly, a study involving 237 STEMI patients undergoing primary PCI reported a GV rate of 33%, defined as MAGE > 66 mg/dL within the first 72 h [49]. Notably, in all these studies, patients with higher GV were more likely to have diabetes, receive insulin treatment for acute hyperglycemia, and consequently present with higher admission glucose levels. However, when only patients with STEMI without diabetes were considered, a MAGE > 60 mg/dL during the first 72 h was observed in 34% of them [50].
Prognostic relevance of GV in AMI
In the context of AMI, Su et al. [48] investigated the correlation between GV, determined by CGM systems, and a composite endpoint of cardiovascular death, recurrent AMI, and acute heart failure at 1 year. Patients with a MAGE ≥ 70 mg/dL had a threefold higher rate of this composite endpoint compared to those with a MAGE below this threshold (24% vs. 8%). Elevated MAGE levels predicted major cardiovascular events (hazard ratio 2.4) even after adjusting for the Global Registry of Acute Coronary Events (GRACE) risk score. Another study examined the association between GV and mid-term major cardiac events (median follow-up 17 months) in 327 patients with diabetes hospitalized with acute coronary syndromes [51]. Interestingly, increased GV, defined as a standard deviation of glycemia ≥ 49 mg/dL and assessed during hospitalization using point-of-care measurement of capillary glucose values, emerged as the strongest independent predictor of cardiovascular death, recurrent AMI, and acute heart failure (odds ratio [OR] 2.2), surpassing even left ventricular ejection fraction < 40% (OR 1.7) and a GRACE score > 140 (OR 1.1). However, some previous studies have failed to establish a clear association between GV and outcomes in patients with AMI. In a re-analysis of the Hyperglycemia and Its Effect After Acute Myocardial Infarction on Cardiovascular Outcomes in Patients with Type 2 Diabetes (HEART2D) study, Siegelaar et al. [52] demonstrated that a reduction in GV through insulin treatment did not lower cardiovascular events in patients with diabetes hospitalized with AMI. Additionally, a report from the DIGAMI-2 trial exploring the prognostic implications of GV during the first 48 h of hospitalization for AMI in patients with diabetes receiving insulin-glucose infusion found no correlation between GV and the 1-year risk of the composite endpoint of death, re-infarction, or stroke [53].
Zhang et al. [49] observed in 237 STEMI patients monitored by CGM systems for 72 h after primary PCI that MAGE levels correlated with enzymatic infarct size and the incidence of in-hospital cardiac death, re-infarction, repeat target vessel revascularization, or recurrent angina. Thus, accumulating evidence suggests that elevated GV should be considered a prognostic parameter for short-term and long-term risk stratification of patients hospitalized with AMI. Another study that enrolled 417 patients with acute coronary syndrome analyzed glycemic variability with CGM. It evaluated MAGE (defined as a variability of plasma glucose level of at least 52 mg/dl) and its relationship with major adverse cardio-cerebral events (MACCEs) showing a higher rate of MACCEs in patients who had wider MAGE; this finding was also confirmed in a multivariate analysis demonstrating that an increased MAGE is an independent predictive factor of poor prognosis [54]. Furthermore, a systematic review that included 11 studies with more than 3,500 patients confirmed that increased GV is related to a poor prognosis in patients with acute coronary syndromes, independently by metrics implied [55].
In 2018, we evaluated acute-to-chronic glycemic ratio at admission in patients with AMI. We measured admission glycemia and estimated the average chronic glucose level based on glycosylated hemoglobin (HbA1c). Our prospective study, using multivariate and reclassification analyses, demonstrated that acute-to-chronic glycemic ratio was a better predictor of in-hospital morbidity and mortality than admission glycemia alone, and it was associated with a parallel increase in peak troponin I values [56]. Furthermore, as noted in a 2021 review, GV continues to significantly impact cardiovascular outcomes [57]. As anticipated by a Delphi consensus, CGM has proven valuable for diagnosis and prognosis in high-risk diabetic patients. The cited studies underscore CGM’s role in detecting GV and supporting better glycemic control [58, 59].
In conclusion, recent evidence supports a strong association between GV and clinical outcomes in patients hospitalized with AMI, suggesting that GV may serve as a valuable prognostic marker. However, it remains unproven whether interventions specifically targeting GV can improve outcomes in this clinical context. Further research is warranted to establish whether reducing GV can yield meaningful clinical benefits in this high-risk population.
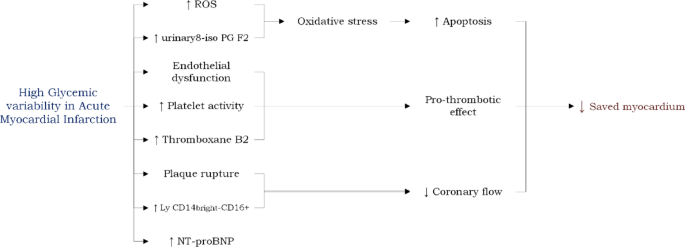
Possible mechanisms underlying the association between high GV and worse prognosis in AMI (Fig. 2). In the past two decades, experimental studies have shed light on the detrimental effects of GV. In vitro exposure of human umbilical vein endothelial cells to both constant and intermittent high glucose mediums has been shown to stimulate the overproduction of reactive oxygen species (ROS) by the mitochondrial respiratory chain. The resulting heightened oxidative stress and increased cellular apoptosis were more pronounced when the culture was exposed to intermittent, rather than constant, high glucose medium. Interestingly, at the same level of hyperglycemia, the severity of endothelial dysfunction and oxidative stress were directly correlated with the magnitude of glucose level increase [60]. Clinical studies have also corroborated these findings. For instance, acute glucose fluctuations, but not sustained hyperglycemia, have been positively correlated with the urinary excretion rate of free-8-isoprostaglandine F2, a marker of oxidative stress, in patients with type-2 diabetes treated with oral anti-hyperglycemic agents [61]. Similarly, oscillating glucose over a 24-h period was found to be more damaging to endothelial function than stable constant high glucose, irrespective of diabetic status [62]. Interestingly, a study involving 37 consecutive patients with AMI who underwent optical coherence tomography (OCT) revealed plaque rupture in 24 patients and no rupture in 13 at the culprit coronary site. The study found that MAGE, measured via CGM, was significantly higher in patients with plaque rupture compared to those without. Additionally, levels of a specific monocyte sub-population (CD14brightCD16+) were significantly elevated in the rupture group. Notably, the prevalence of this monocyte subset showed a significant positive correlation with MAGE [63].
Biochemical and clinical effects of high glycemic variability in acute myocardial infarction. Ly Lymphocyte, NT-proBNP N-terminal pro B-type Natriuretic Peptide, ROS Reactive oxygen species; urinary 8 isoPG F2 = urinary 8 iso-prostaglandin F2
However, no relationship was found between GV and oxidative stress markers in a small cohort of patients with type 1 diabetes [64]. In a separate small observational study, an increased mean daily difference in blood glucose blood levels was directly correlated with increased NT-proBNP levels, although this association has not yet been linked to adverse clinical outcomes [65].
Experimental models of diabetic heart disease have demonstrated that glucose fluctuations exacerbate ischemia/reperfusion injury by increasing the generation of ROS [66]. Clinical evidence supports these findings. In patients with AMI, high GV has been associated with electrocardiographic and angiographic indicators of impaired myocardial reperfusion [67]. For instance, in STEMI patients undergoing primary PCI and monitored by CGM systems, a lower rate of electrocardiographic ST-segment resolution was observed in those with high GV. Moreover, high GV was linked to angiographic no-reflow and lower myocardial blush grades in STEMI patients treated with primary PCI [54]. Additionally, a negative correlation was found between high GV and the myocardial salvage index, as assessed by cardiac magnetic resonance imaging, in reperfused STEMI patients [68].
Another potential mechanism linking GV to outcomes in patients with AMI involves platelet activity. A recent study in patients with type 2 diabetes on clopidogrel therapy demonstrated that those with high residual platelet activity also exhibit higher GV [69]. Additionally, high GV has been associated with increased production of thromboxane B2, a marker of in-vivo platelet activation [70]. These findings suggest a greater propensity for thrombotic complications in patients with AMI with elevated GV.
Gaps in knowledge and future perspectives
Despite the well-established link between persistent high GV and an increased occurrence of both macrovascular and microvascular complications in patients with diabetes, the precise role of GV during the acute phase of AMI remains incompletely understood.
Although international consensus reports [46, 71] recommend standardized metrics for assessing short-term GV, their limited use in research and clinical practice, coupled with an incomplete understanding of GV’s biological effects on ischemic myocardium, continue to challenge the establishment of a clear causal link between GV and clinical outcomes in AMI. Moreover, it is uncertain how GV during the acute phase of AMI contributes, in terms of prognostic significance, compared to acute hyperglycemia. Notably, as acute hyperglycemia typically resolves promptly with insulin therapy during AMI, patients with the highest blood glucose levels upon hospital admission also tend to experience the greatest variability in glucose levels during normalization. Thus, GV in the initial hours of AMI may reflect the flip side of stress-induced hyperglycemia. The complexity is further compounded by the observation that elevated blood glucose levels at admission may not necessarily indicate true stress hyperglycemia, particularly in individuals with diabetes who may already have chronically elevated glucose levels. This ambiguity makes it challenging to determine the independent prognostic role of GV in this clinical context. Particularly in patients with diabetes, the swift correction of chronically elevated glucose levels may inadvertently lead to iatrogenic increases in GV, potentially resulting in adverse clinical outcomes.
In this regard, hypoglycemia represents another underappreciated but harmful facet of glucose dysregulation during AMI. Episodes of hypoglycemia, particularly when occurring as a consequence of aggressive glucose-lowering strategies, may exert proarrhythmic effects, trigger sympathoadrenal activation, and exacerbate myocardial ischemia. The prognostic implications of hypoglycemia—especially when superimposed on a background of high GV—remain largely undefined in the context of AMI. It is also unclear whether the cardiovascular risk associated with hypoglycemia differs between spontaneous and iatrogenic episodes, and how such events interact with the overall glycemic profile during hospitalization. Additionally, the detection of hypoglycemia is often limited by intermittent glucose monitoring, potentially leading to underestimation of its frequency and clinical impact. These gaps in evidence further highlight the need for CGM and refined clinical protocols to identify and mitigate harmful glycemic excursions on both ends of the spectrum. To this end, metrics like MAGE, by capturing both high and low glucose swings, may better inform pharmacological strategies particularly when aiming to minimize hypoglycemic risk without compromising glycemic control. Therefore, GV, through MAGE, might be more effective in tailoring individualized treatment plans.
Nevertheless, some studies have identified GV as an independent predictor of outcomes in patients with AMI, even after accounting for admission glycemia as a confounding factor, suggesting an additional prognostic contribution. However, this observation warrants confirmation, particularly when assessing “true” stress hyperglycemia rather than solely relying on the absolute glycemic value at admission. There are two ongoing clinical trials focused on exploring the prognostic role of GV in patients with AMI, according to available data from ClinicalTrials.gov as of the latest update on May 19th, 2025. The characteristics of these two studies are summarized in Table 1. Future research is essential to definitively clarify the clinical significance of high GV and its therapeutic management in patients with AMI.
Finally, while hypoglycemia and GV are increasingly recognized as significant prognostic factors in AMI, their targeted management remains underexplored. Current treatment strategies largely focus on hyperglycemia control, often neglecting the harmful effects of glucose excursions on both ends of the spectrum. Among the potential interventions, glucose-insulin-potassium (GIK) infusions have been extensively studied. GIK was initially proposed to provide metabolic support to the ischemic myocardium by optimizing substrate utilization, limiting infarct size, and minimizing GV. Early trials showed promising results, particularly when administered early after symptom onset [72]. However, subsequent large randomized studies, including the CREATE-ECLA trial, failed to demonstrate a consistent benefit in reducing mortality or major cardiac events [73]. Moreover, concerns about fluid overload and hypoglycemia have limited its widespread adoption. In parallel, glucagon-like peptide-1 (GLP-1) receptor agonists have gained attention due to their cardioprotective properties and low risk of inducing hypoglycemia. Experimental studies have suggested that GLP-1 agonists may improve left ventricular function post-AMI and reduce infarct size, particularly when administered acutely [74]. Their ability to attenuate GV without triggering hypoglycemic episodes adds to their therapeutic appeal in this setting. For example, a study by Read et al. [75] showed that GLP-1 infusion is able to reduce ischemic left ventricular dysfunction after supply ischemia during coronary balloon occlusion in patients undergoing elective PCI and mitigates stunning. Given the complex interplay between glycemic control and cardiovascular outcomes, further investigation is warranted to determine whether strategies like GIK or GLP-1 agonist therapy can meaningfully reduce hypoglycemia, GV, and their associated risks. Integrating such targeted approaches into AMI treatment protocols may ultimately improve both short- and long-term patient outcomes.