Larvicidal activity
At 24 h, no mortality rate (p˃0.05) was recorded across the different extracts with 0.5% (v/v) extract dose compared to the control. At 48 and 72 h, there was a significant (p < 0.05) mortality rate compared to the control. The increase in mortality was time-dependent, with more deaths occurring at 72 h (Fig. 2A). With a 1% (v/v) dose of the extracts, at 24 h, the combined extracts showed a significant (p < 0.05) mortality rate compared to the control, whereas, at 24 and 72 h, all the extracts showed a significant mortality rate compared to the control (Fig. 2B). With 1.5% (v/v) dose of the extracts, clove extract, garlic extract, and combined extracts, respectively, showed a significant mortality rate compared to the control (Fig. 2C). The calculated LC 50 and LC 90 after 24 h (Table 1) are 1.824 and 2.453 for garlic extract, 2.315% and 3.257% for clove extract, and 0.994 and 1.414 for the combined extracts.
Larvicidal effect of clove/garlic hydro-ethanolic extracts at 0.5% B, 1.5% C, and 1.5% A (v/v). Data is shown as mean ± SEM of 5 replicates (n = 20). Bars with superscripts a and b are significantly different from bars with superscript # at p < 0.05
Adulticidal activity
At 10 min, there was no significant knockdown rate in adult Anopheles mosquitoes by clove extract compared to deltamethrin, but the combined extract showed a significant (p < 0.05) knockdown rate. At 15, 20, 30, 40, 50, and 60 min, the clove extract and combined extracts showed a significant knockdown rate of adult mosquitoes compared to deltamethrin. The knockdown effects between 15 to 60 min were time-dependent (Fig. 3). Garlic extract did not show any knockdown effects on adult mosquitoes. The mortality rate of adult mosquitoes was significantly increased (p < 0.05) by clove extract and combined extracts of clove and garlic compared to deltamethrin (Fig. 4). The garlic extract showed no mortality rate compared to deltamethrin (Fig. 4).
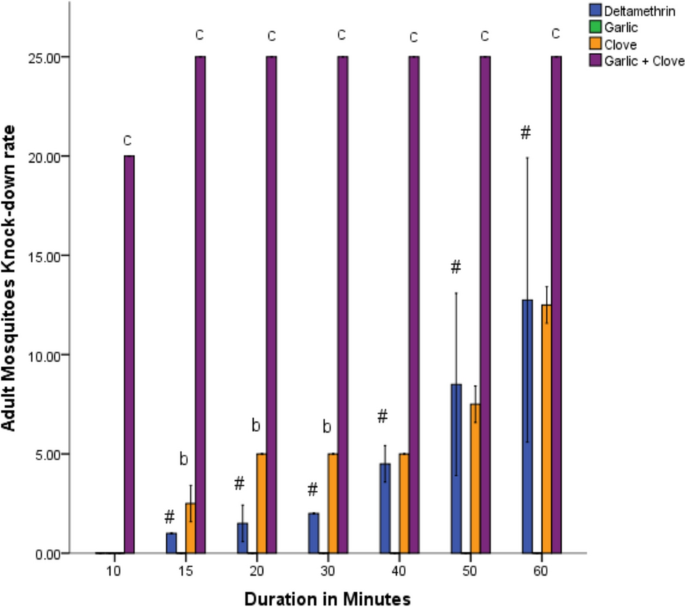
Knocked-down effect of the extracts on adult mosquitoes after 60 min. Data is shown as mean ± SEM of four replicates (n = 25). Bars with varying superscripts from deltamethrin (standard) exhibit a significant difference at p < 0.05
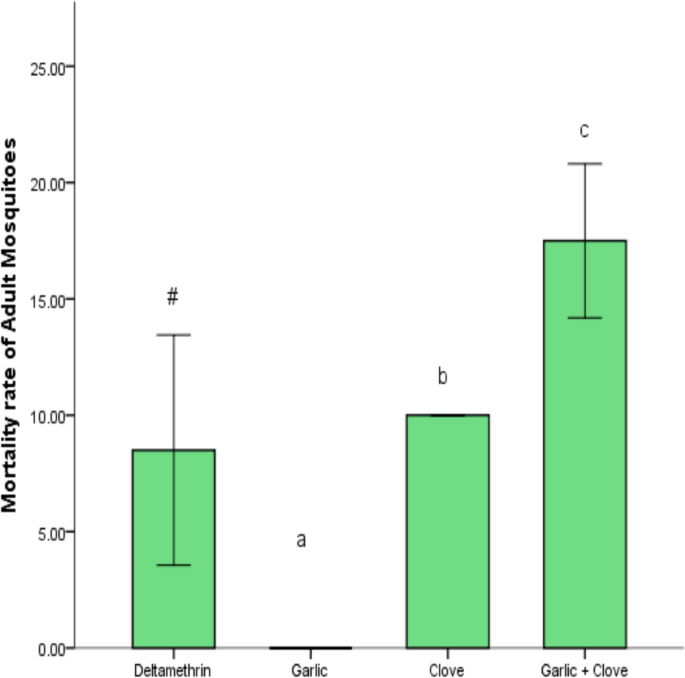
Effect of extracts on mortality rate of adult mosquitoes. Data is shown as mean ± SEM of four replicates (n = 25). Bars with varying superscripts from deltamethrin (standard) exhibit a significant difference at p < 0.05
Enzyme analysis
There was a significant increase (p < 0.05) in potassium ion concentration in groups treated with clove extract, garlic extract, and combined extract compared to that in the deltamethrin group (Fig. 5A). Sodium ion concentration was significantly decreased (p < 0.05) in the group treated with clove, garlic, and combined extract compared to deltamethrin (Fig. 5B). There was a significant increase (p < 0.05) in acetylcholine activity in groups administered clove, garlic, and the combined extract compared to deltamethrin (Fig. 6A). Similarly, there was a significant increase (p < 0.05) in Na–k ATPase activity in groups administered clove, garlic, and the combined extract compared to deltamethrin (Fig. 6B). There was a significant decrease (p < 0.05) in glutathione-transferase activity in the group treated with garlic extract and combined extracts of clove and garlic compared to deltamethrin. In contrast, no significant change was observed in the enzyme activity in the clove-treated group (Fig. 7A). There was a significant decrease (p < 0.05) in protein concentration in the groups treated with clove and garlic extracts compared to the group treated with deltamethrin. There was no significant change in the group treated with the combined extracts (Fig. 7B).
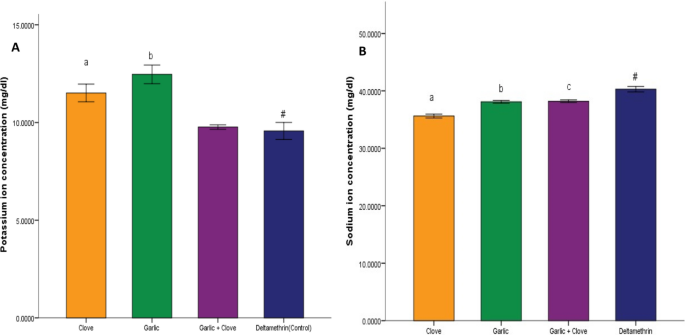
Effect of clove/garlic hydroethanolic extracts on potassium ion concentration (A) and sodium ion concentration (B) in adult mosquitoes (Anopheles gambiae). Data is shown as mean ± SEM of four replicates (n = 25). Bars with superscripts a and b exhibit a significant difference from bar with superscript # at p < 0.05
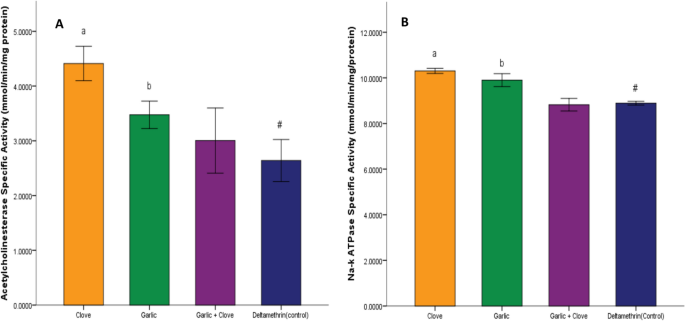
Effect of clove/garlic hydro-ethanolic extracts on acetylcholinesterase activity (A) and Na–K ATPase activity (B) in adult mosquitoes (Anopheles gambiae). Data is shown as mean ± SEM of four replicates (n = 25). Bars with superscripts a and b exhibit a significant difference from bar with superscript # at p < 0.05
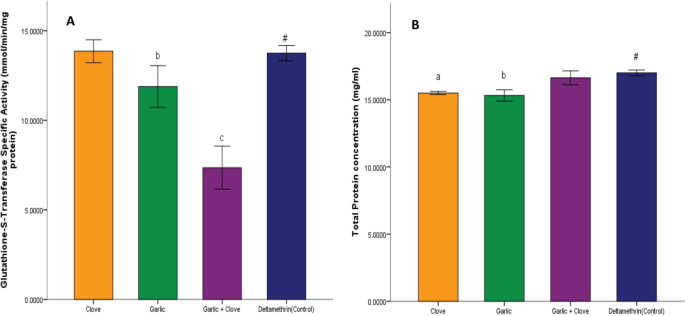
Effect of clove/garlic hydro-ethanolic extracts on glutathione-S-transferase activity in adult mosquitoes (Anopheles gambiae). Data is shown as mean ± SEM of four replicates (n = 25). Bars with superscripts b and c exhibit a significant difference from bar with superscript # at p < 0.05
Phytochemical analysis
The quantitative phytochemical evaluation shows that saponin and flavonoids are present in the garlic while saponin, tannins, phenols, and steroids are in the clove. In contrast, saponin, tannins and phenols, flavonoids, and steroids were present in the ratio 1:1 mixture of the garlic and clove (Table 2). The chromatogram of GC–MS evaluation of clove, garlic, and the combination of clove and garlic shows the presence of different compounds at different abundance and retention times (Figs. 8A–C). The GC–MS analysis of clove hydro-ethanolic extract revealed the presence of 16 compounds (Table 3) while the GC–MS constituent of garlic and clove-garlic combination showed 18 and 23 compounds respectively (Tables 4 & 5).
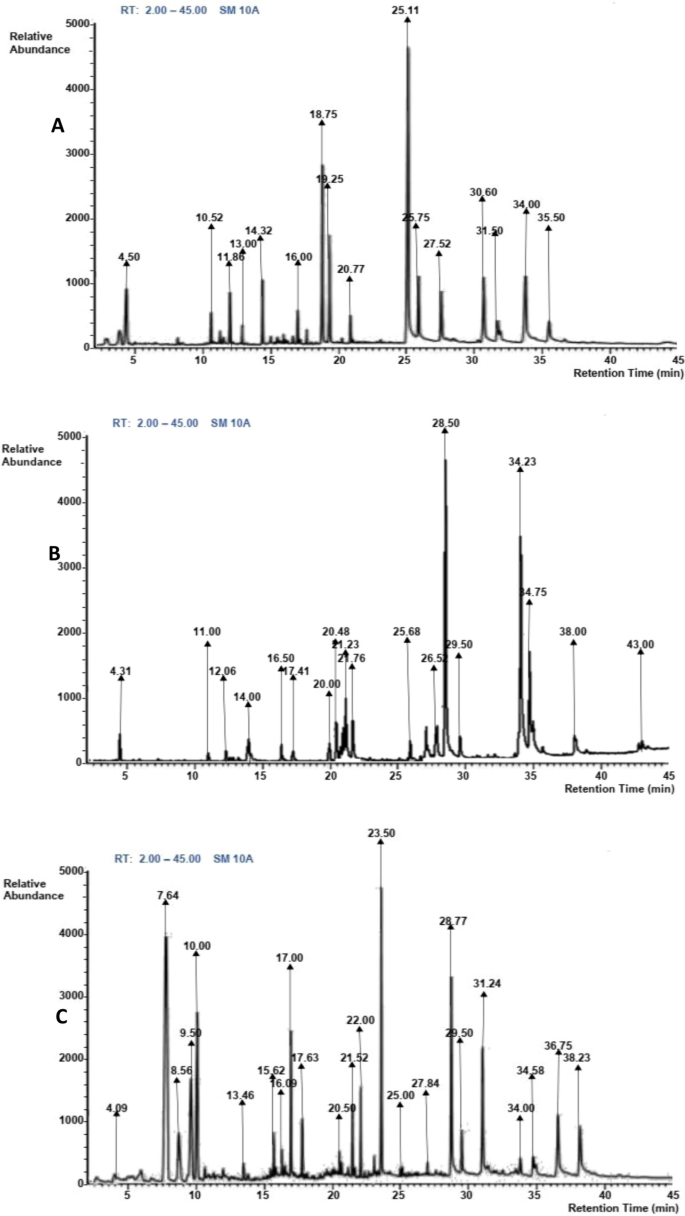
A typical gas chromatogram of the chemical compounds of clove hydro-ethanolic extracts (A), garlic hydro-ethanolic extract (B), and garlic and clove hydro-ethanolic extracts (C)
Molecular docking
Predicted upon their binding affinities falling below the threshold of − 8.0 kcal/mol for acetylcholinesterase and − 7.0 kcal/mol for voltage-gated sodium channels, several ligands were singled out for exclusion, as evidenced by the molecular docking outcomes meticulously depicted in Tables 6, 7, 8. However, four compounds from both proteins manifest binding affinities that are higher or close to that of the reference standard (− 8.0 to − 9.4 kcal/mol). Estrone has higher binding affinities (− 9.4 kcal/mol) with both proteins than the standard drug. The conformation of acetylcholinesterase (PDB ID: 5YDH) and the Voltage-gated sodium channels (PDB ID: 6MVVA) were assessed using a Ramachandran plot (Fig. 9A–B), which revealed that 99% and 98% of their residues respectively reside within the favoured allowed regions. The target active site of acetylcholinesterase protein was found to contain amino acid residues, which include Phe490, Tyr493, and Gly44 while, that of acetylcholinesterase was identified to contain Phe1107, Val1110, Val1120, Phe1079, Trp1076 and Pro1075 as major residues involved in interactions. These were obtained from a reverse docking of the native ligand of the acetylcholinesterase complex (2-acetamido-2-deoxy-beta-D-glucopyranose (NAG)) and that of the voltage-gated sodium channels (2-Dimyristoyl-sn-glycero-3-phosphocholine) (Table 9).
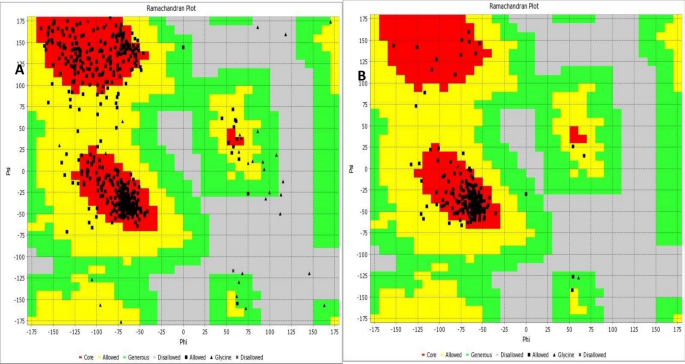
Ramachandran plot of the acetylcholinesterase (PDB ID: 5YDHA) showing 99% residues in core/allowed region and Ramachandran plot of the Voltage-gated ion channel (PDB ID: 6MVVA) showing 98% residues in core/allowed region
Binding mode and molecular interactions of the best hit compound and the standard
During the lead optimization stage of drug discovery, the binding mode and molecular interactions involved in ligand binding to the target receptor’s active site are critical. It helps to increase the chosen hit compounds’ potency and effectiveness. A comparison was made between the optimal Hit compounds’ molecular interactions (estrone, morellinol, caryophyllene, 1,4,7-cycloundecatriene, 1,5,9,9-tetramethyl-, Z, Z, Z-), the binding mode, and the standard reference compound (deltamethrin) for both proteins. It is noteworthy that these compounds had similar binding affinities and inhibition efficiency against the proteins. The target proteins were found to contain, among other amino residues, Phe490, Tyr493, and Gly445 (for PDB ID: 5YDH) and Phe1107, Val1110, Val1120, Phe1079, Trp1076, and Pro1075 (for PDB ID: 6MVV), according to the reverse docking analysis. Table 4 showed the interaction of estrone, morellinol, caryophyllene, 1,4,7-cycloundecatriene,1,5,9,9-tetramethyl-, Z, Z, Z- and the reference compound (deltamethrin) alongside the target’s binding site of Anopheles’ acetylcholinesterase shows that estrone formed an alkyl bond with the target receptor through Tyr282, Trp441, Val235, Tyr493, Phe490. Through the formation of a conventional hydrogen bond, morellinol and the receptor Ala391, Ser397, Pro352, Tyr558, Pro521, an alkyl bond through Val396, His559, and a Pi-Pi T shape through Tyr522. Caryophyllene also interacted with the protein to form Alkyl bonds through Tyr493, Tyr282, Ile446, Phe490, Tyr494, and the Pi-sigma bond through Trp441. 1,4,7- cycloundecatriene,1,5,9,9-tetramethyl-, Z, Z, Z- form a similar Pi-Alkyl bond with the target receptor through Phe449, Trp441, Tyr493, Tyr489. Similar to this, the selected reference compound binds to the protein through Ser283 to form a conventional hydrogen bond and through Tyr282, Ile231, Tyr494, and Asp233 to form an alkyl bond. Table 4 clearly shows how precisely the target receptor was contacted by estrone, caryophyllene, 1,4,7-cycloundecatriene, 1,5,9,9-tetramethyl-, Z, Z, Z-, and other chemicals through the amino residues that were found to be present at the active site. Nevertheless, none of the identified amino acid residues allowed the reference compound to interact with the active site of acetylcholinesterase. Using voltage-gated sodium channels Trp1076, Val1120, and Val1110, Estrone formed an alkyl bond with the target receptor. For these channels, it also formed a Pi-sigma bond through Phe1107 and a Pi-Pi shapes bond through Phe1079 for these channels. Morellinol and the receptor bonded via an alkyl bond through Val1110, Leu1104, and Val1120. It created Pi-sigma bonds through Phe1107, Pi-Pi stacked bonds through Phe1079, and Pro1075 also revealed an unfavourable bond. Caryophyllene also interacted with the active site to form Alkyl bonds through Trp1076, Val1110, Val1120. It formed a weak van der Waal’s bond through Thr1111 and Phe1107 and lastly a Pi-sigma bond through Phe1079. 1,4,7 Cycloundecatriene,1,5,9,9- tetramethyl-, Z, Z, Z- also formed a similar weak van der Waal’s bond through Thr1111, alkyl bond with the target receptor through Phe1079, Val1110, Trp1076, Pro1075, Val1120, Ile1124. Similarly, the selected reference compound formed bonds with the target protein through Phe1079, Trp1116, Pro1076, Phe1107, Val1110 and Val1120. Binding mode and binding interaction of the hit’s ligands and reference compound with acetylcholinesterase and Voltage-gated sodium channels respectively (Table 7 and 9).
