Lee H, Halverson S, Ezinwa N. Mosquito-borne diseases. Prim Care. 2018;45:393–407.
Wong JM, Adams LE, Durbin AP, Munoz-Jordan JL, Poehling KA, Sanchez-Gonzalez LM, et al. Dengue: a growing problem with new interventions. Pediatrics. 2022;149:e2021055522.
Monath TP, Vasconcelos PF. Yellow fever. J Clin Virol. 2015;64:160–73.
White NJ. Severe malaria. Malar J. 2022;21:284.
WHO. World malaria report. 2023.
Kularatne SA, Dalugama C. Dengue infection: global importance, immunopathology and management. Clin Med. 2022;22:9–13.
Pajor MJ, Long B, Liang SY. Dengue: a focused review for the emergency clinician. Am J Emerg Med. 2024;82:82–7.
Cracknell DB, Gaythorpe K, Imai N, Dorigatti I. Yellow fever in Asia–a risk analysis. J Travel Med. 2021;28:taab015.
Jones RT, Ant TH, Cameron MM, Logan JG. Novel control strategies for mosquito-borne diseases. Philos Trans R Soc Lond B Biol Sci. 1818;2021:20190802.
Ni J, Wang J, Fang C, Zhang W, Gong Z. A review of the latest control strategies for mosquito-borne diseases. China Cdc Wkly. 2024;6:852–6.
Onen H, Luzala MM, Kigozi S, Sikumbili RM, Muanga CK, Zola EN, et al. Mosquito-borne diseases and their control strategies: an overview focused on green synthesized plant-based metallic nanoparticles. Insects. 2023;14:221.
Sun R, Raban R, Akbari OS. CRISPR-Cas9 methods and key considerations in the production of Aedes aegypti mutant strains. Cold Spring Harb Protoc. 2023;2023:607–13.
Sabet A, Goddard J. Promise or peril: using genetically modified mosquitoes in the fight against vector-borne disease. Am J Med. 2022;135:281–3.
Bernardini F, Haghighat-Khah RE, Galizi R, Hammond AM, Nolan T, Crisanti A. Molecular tools and genetic markers for the generation of transgenic sexing strains in anopheline mosquitoes. Parasit Vectors. 2018;11:660.
Foster WA. Mosquito sugar feeding and reproductive energetics. Annu Rev Entomol. 1995;40:443–74.
Pitts RJ, Liu C, Zhou X, Malpartida JC, Zwiebel LJ. Odorant receptor-mediated sperm activation in disease vector mosquitoes. Proc Natl Acad Sci USA. 2014;111:2566–71.
Coutinho-Abreu IV, Riffell JA, Akbari OS. Human attractive cues and mosquito host-seeking behavior. Trends Parasitol. 2022;38:246–64.
David OG, Sanchez KM, Arce AV, Costa-Da-Silva AL, Bellantuono AJ, Degennaro M. Fertility decline in female mosquitoes is regulated by the orco olfactory co-receptor. Iscience. 2023;26:106883.
Alvarez-Ocana R, Shahandeh MP, Ray V, Auer TO, Gompel N, Benton R. Odor-regulated oviposition behavior in an ecological specialist. Nat Commun. 2023;14:3041.
Suh E, Choe DH, Saveer AM, Zwiebel LJ. Suboptimal larval habitats modulate oviposition of the malaria vector mosquito Anopheles coluzzii. PLoS ONE. 2016;11:e149800.
Saveer AM, Pitts RJ, Ferguson ST, Zwiebel LJ. Characterization of chemosensory responses on the labellum of the malaria vector mosquito, Anopheles coluzzii. Sci Rep. 2018;8:5656.
Suh E, Bohbot J, Zwiebel LJ. Peripheral olfactory signaling in insects. Curr Opin Insect Sci. 2014;6:86–92.
Ignell R, Dekker T, Ghaninia M, Hansson BS. Neuronal architecture of the mosquito deutocerebrum. J Comp Neurol. 2005;493:207–40.
Konopka JK, Task D, Poinapen D, Potter CJ. Neurogenetic identification of mosquito sensory neurons. Iscience. 2023;26:106690.
Thomas A, Roy M, Gupta N. Olfactory coding in the mosquito antennal lobe: labeled lines or combinatorial code? Curr Opin Insect Sci. 2025;68:101299.
Riabinina O, Task D, Marr E, Lin CC, Alford R, O’Brochta DA, et al. Organization of olfactory centres in the malaria mosquito Anopheles gambiae. Nat Commun. 2016;7:13010.
Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15:1535–47.
Herre M, Goldman OV, Lu TC, Caballero-Vidal G, Qi Y, Gilbert ZN, et al. Non-canonical odor coding in the mosquito. Cell. 2022;185:3104–23.
Wheelwright M, Whittle CR, Riabinina O. Olfactory systems across mosquito species. Cell Tissue Res. 2021;383:75–90.
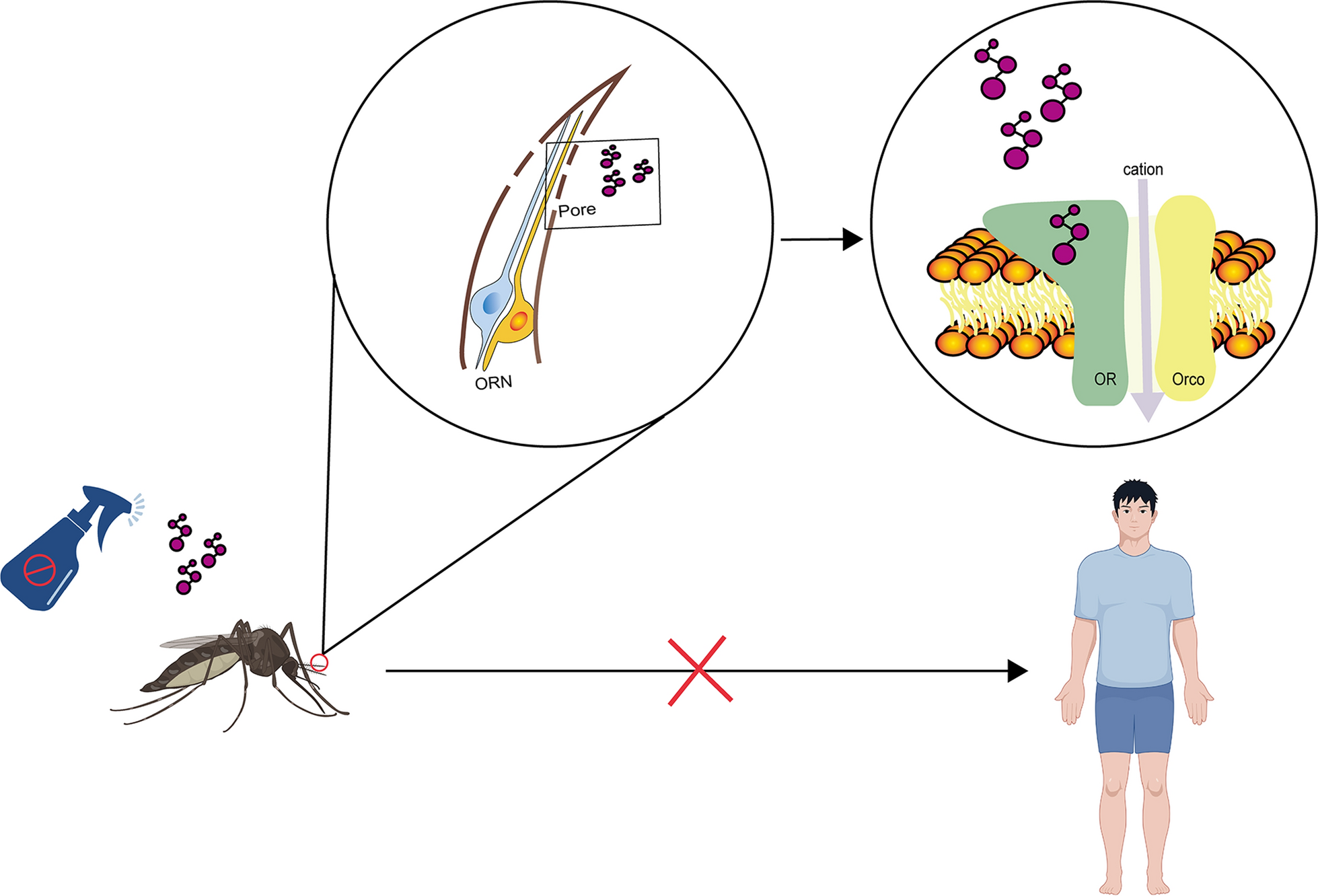
Sparks JT, Botsko G, Swale DR, Boland LM, Patel SS, Dickens JC. Membrane proteins mediating reception and transduction in chemosensory neurons in mosquitoes. Front Physiol. 2018;9:1309.
Pask GM, Jones PL, Rutzler M, Rinker DC, Zwiebel LJ. Heteromeric anopheline odorant receptors exhibit distinct channel properties. PLoS ONE. 2011;6:e28774.
He Z, Yu Z, He X, Hao Y, Qiao L, Luo S, et al. Genome-wide identification and expression profiling of odorant receptor genes in the malaria vector Anopheles sinensis. Parasit Vectors. 2022;15:143.
Arensburger P, Megy K, Waterhouse RM, Abrudan J, Amedeo P, Antelo B, et al. Sequencing of Culex quinquefasciatus establishes a platform for mosquito comparative genomics. Science. 2010;330:86–8.
Hill CA, Fox AN, Pitts RJ, Kent LB, Tan PL, Chrystal MA, et al. G protein-coupled receptors in Anopheles gambiae. Science. 2002;298:176–8.
Bohbot J, Pitts RJ, Kwon HW, Rutzler M, Robertson HM, Zwiebel LJ. Molecular characterization of the Aedes aegypti odorant receptor gene family. Insect Mol Biol. 2007;16:525–37.
Lombardo F, Salvemini M, Fiorillo C, Nolan T, Zwiebel LJ, Ribeiro JM, et al. Deciphering the olfactory repertoire of the tiger mosquito Aedes albopictus. BMC Genomics. 2017;18:770.
Mier P, Fontaine JF, Stoldt M, Libbrecht R, Martelli C, Foitzik S, et al. Annotation and analysis of 3902 odorant receptor protein sequences from 21 insect species provide insights into the evolution of odorant receptor gene families in solitary and social insects. Genes (Basel). 2022;13:919.
Yan H, Jafari S, Pask G, Zhou X, Reinberg D, Desplan C. Evolution, developmental expression and function of odorant receptors in insects. J Exp Biol. 2020;223:jeb208215.
Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–62.
Knecht ZA, Silbering AF, Ni L, Klein M, Budelli G, Bell R, et al. Distinct combinations of variant ionotropic glutamate receptors mediate thermosensation and hygrosensation in Drosophila. Elife. 2016;5:e17879.
Rytz R, Croset V, Benton R. Ionotropic receptors (IRs): chemosensory ionotropic glutamate receptors in Drosophila and beyond. Insect Biochem Mol Biol. 2013;43:888–97.
Abuin L, Bargeton B, Ulbrich MH, Isacoff EY, Kellenberger S, Benton R. Functional architecture of olfactory ionotropic glutamate receptors. Neuron. 2011;69:44–60.
Silbering AF, Rytz R, Grosjean Y, Abuin L, Ramdya P, Jefferis GS, et al. Complementary function and integrated wiring of the evolutionarily distinct Drosophila olfactory subsystems. J Neurosci. 2011;31:13357–75.
Sparks JT, Vinyard BT, Dickens JC. Gustatory receptor expression in the labella and tarsi of Aedes aegypti. Insect Biochem Mol Biol. 2013;43:1161–71.
Kent LB, Walden KK, Robertson HM. The Gr family of candidate gustatory and olfactory receptors in the yellow-fever mosquito Aedes aegypti. Chem Senses. 2008;33:79–93.
Ma D, Hu M, Yang X, Liu Q, Ye F, Cai W, et al. Structural basis for sugar perception by Drosophila gustatory receptors. Science. 2024;383:eadj2609.
Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90.
Robertson HM, Kent LB. Evolution of the gene lineage encoding the carbon dioxide receptor in insects. J Insect Sci. 2009;9:19.
Kohl J, Huoviala P, Jefferis GS. Pheromone processing in Drosophila. Curr Opin Neurobiol. 2015;34:149–57.
Jiang JZ, Xiao Q, Gao ZL, Gu J, Huang LH. Identification and function analysis of two new gustatory receptors related to silkworm monophagy. Insect Sci. 2025. https://doi.org/10.1111/1744-7917.70006.
Erdelyan CN, Mahood TH, Bader TS, Whyard S. Functional validation of the carbon dioxide receptor genes in Aedes aegypti mosquitoes using RNA interference. Insect Mol Biol. 2012;21:119–27.
Robertson HM. Molecular evolution of the major arthropod chemoreceptor gene families. Annu Rev Entomol. 2019;64:227–42.
Hinze A, Pelletier J, Ghaninia M, Marois E, Hill SR, Ignell R. Knockout of or39 reveals redundancy in the olfactory pathway regulating the acquisition of host seeking in Anopheles coluzzii. Proc Biol Sci. 2011;2023:20232092.
Omondi AB, Ghaninia M, Dawit M, Svensson T, Ignell R. Age-dependent regulation of host seeking in Anopheles coluzzii. Sci Rep. 2019;9:9699.
Gu ZY, Gao HT, Yang QJ, Ni M, Li MJ, Xing D, et al. Screening of olfactory genes related to blood-feeding behaviors in Culex pipiens quinquefasciatus and Culex pipiens molestus by transcriptome analysis. Plos Negl Trop Dis. 2022;16:e10204.
Ni M, Zhao T, Lv HX, Li MJ, Xing D, Zhao TY, et al. Screening for odorant receptor genes expressed in Aedes aegypti involved in host-seeking, blood-feeding and oviposition behaviors. Parasit Vectors. 2022;15:71.
Taparia T, Ignell R, Hill SR. Blood meal induced regulation of the chemosensory gene repertoire in the southern house mosquito. BMC Genomics. 2017;18:393.
Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–60.
Pullmann-Lindsley H, Huff RM, Boyi J, Pitts RJ. Odorant receptors for floral- and plant-derived volatiles in the yellow fever mosquito, Aedes aegypti (Diptera: Culicidae). PLoS ONE. 2024;19:e302496.
Wang X, Chen Z, Wang Y, Liu F, Jiang S, Liu N. Heterologous expression and functional analysis of Aedes aegypti odorant receptors to human odors in Xenopus oocytes. J Vis Exp. 2021;172:e61813.
Dekel A, Pitts RJ, Yakir E, Bohbot JD. Evolutionarily conserved odorant receptor function questions ecological context of octenol role in mosquitoes. Sci Rep. 2016;6:37330.
Lu T, Qiu YT, Wang G, Kwon JY, Rutzler M, Kwon HW, et al. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Curr Biol. 2007;17:1533–44.
Dormont L, Mulatier M, Carrasco D, Cohuet A. Mosquito attractants. J Chem Ecol. 2021;47:351–93.
Takken W, Kline DL. Carbon dioxide and 1-octen-3-ol as mosquito attractants. J Am Mosq Control Assoc. 1989;5:311–6.
Mappin F, Bellantuono AJ, Ebrahimi B, Degennaro M. Odor-evoked transcriptomics of Aedes aegypti mosquitoes. PLoS ONE. 2023;18:e293018.
Grant AJ, Dickens JC. Functional characterization of the octenol receptor neuron on the maxillary palps of the yellow fever mosquito, Aedes aegypti. PLoS ONE. 2011;6:e21785.
Jung JW, Baeck SJ, Perumalsamy H, Hansson BS, Ahn YJ, Kwon HW. A novel olfactory pathway is essential for fast and efficient blood-feeding in mosquitoes. Sci Rep. 2015;5:13444.
Wu Q, Li CX, Liu QM, Guo XX, Shi QM, Zhang HD, et al. RNA interference of odorant receptor cquior114/117 affects blood-feeding behavior in Culex quinquefasciatus. Acta Trop. 2020;204:105343.
Xu P, Choo YM, Leal WS. Odorant inhibition in mosquito olfaction mediated by inverse agonists. Biochem Biophys Res Commun. 2022;609:156–62.
Pelletier J, Hughes DT, Luetje CW, Leal WS. An odorant receptor from the southern house mosquito Culex pipiens quinquefasciatus sensitive to oviposition attractants. PLoS ONE. 2010;5:e10090.
Hughes DT, Pelletier J, Luetje CW, Leal WS. Odorant receptor from the southern house mosquito narrowly tuned to the oviposition attractant skatole. J Chem Ecol. 2010;36:797–800.
Bohbot JD, Jones PL, Wang G, Pitts RJ, Pask GM, Zwiebel LJ. Conservation of indole responsive odorant receptors in mosquitoes reveals an ancient olfactory trait. Chem Senses. 2011;36:149–60.
Carey AF, Wang G, Su CY, Zwiebel LJ, Carlson JR. Odorant reception in the malaria mosquito Anopheles gambiae. Nature. 2010;464:66–71.
Wang G, Carey AF, Carlson JR, Zwiebel LJ. Molecular basis of odor coding in the malaria vector mosquito Anopheles gambiae. Proc Natl Acad Sci USA. 2010;107:4418–23.
Blackwell A, Johnson SN. Electrophysiological investigation of larval water and potential oviposition chemo-attractants for Anopheles gambiae s.s. Ann Trop Med Parasitol. 2000;94:389–98.
Cork A. Olfactory basis of host location by mosquitoes and other haematophagous diptera. Ciba Found Symp. 1996;200:84–8.
Franco FP, Xu P, Harris BJ, Yarov-Yarovoy V, Leal WS. Single amino acid residue mediates reciprocal specificity in two mosquito odorant receptors. Elife. 2022;11:e82922.
McBride CS, Baier F, Omondi AB, Spitzer SA, Lutomiah J, Sang R, et al. Evolution of mosquito preference for humans linked to an odorant receptor. Nature. 2014;515:222–7.
Vainer Y, Wang Y, Huff RM, Perets D, Sar-Shalom E, Yakir E, et al. A conserved odorant receptor underpins borneol-mediated repellency in culicine mosquitoes. Biorxiv. 2024.
Lu W, Leal WS, Brisco KK, An S, Cornel AJ. A highly expressed odorant receptor from the yellow fever mosquito, aaegor11, responds to (+)- and (-)-fenchone and a phenolic repellent. Insect Biochem Mol Biol. 2022;151:103866.
Yan R, Xu Z, Qian J, Zhou Q, Wu H, Liu Y, et al. Molecular and functional characterization of a conserved odorant receptor from Aedes albopictus. Parasit Vectors. 2022;15:43.
Andreazza F, Valbon W, Dong K. Transfluthrin enhances odorant receptor-mediated spatial repellency in Aedes aegypti. Pestic Biochem Physiol. 2023;192:105387.
Liu F, Wang Q, Xu P, Andreazza F, Valbon WR, Bandason E, et al. A dual-target molecular mechanism of pyrethrum repellency against mosquitoes. Nat Commun. 2021;12:2553.
Huff RM, Pitts RJ. Functional conservation of anopheline linalool receptors through 100 million years of evolution. Chem Senses. 2022;47:bjac032.
Zeng F, Xu P, Leal WS. Odorant receptors from Culex quinquefasciatus and Aedes aegypti sensitive to floral compounds. Insect Biochem Mol Biol. 2019;113:103213.
Xu P, Choo YM, De La Rosa A, Leal WS. Mosquito odorant receptor for DEET and methyl jasmonate. Proc Natl Acad Sci USA. 2014;111:16592–7.
Xu P, Zeng F, Bedoukian RH, Leal WS. Deet and other repellents are inhibitors of mosquito odorant receptors for oviposition attractants. Insect Biochem Mol Biol. 2019;113:103224.
Clyne PJ, Warr CG, Freeman MR, Lessing D, Kim J, Carlson JR. A novel family of divergent seven-transmembrane proteins: candidate odorant receptors in Drosophila. Neuron. 1999;22:327–38.
Vosshall LB, Amrein H, Morozov PS, Rzhetsky A, Axel R. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell. 1999;96:725–36.
Wicher D, Schafer R, Bauernfeind R, Stensmyr MC, Heller R, Heinemann SH, et al. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452:1007–11.
Tsitoura P, Andronopoulou E, Tsikou D, Agalou A, Papakonstantinou MP, Kotzia GA, et al. Expression and membrane topology of Anopheles gambiae odorant receptors in lepidopteran insect cells. PLoS ONE. 2010;5:e15428.
Smart R, Kiely A, Beale M, Vargas E, Carraher C, Kralicek AV, et al. Drosophila odorant receptors are novel seven transmembrane domain proteins that can signal independently of heterotrimeric G proteins. Insect Biochem Mol Biol. 2008;38:770–80.
Turner RM, Derryberry SL, Kumar BN, Brittain T, Zwiebel LJ, Newcomb RD, et al. Mutational analysis of cysteine residues of the insect odorant co-receptor (orco) from Drosophila melanogaster reveals differential effects on agonist- and odorant-tuning receptor-dependent activation. J Biol Chem. 2014;289:31837–45.
German PF, van der Poel S, Carraher C, Kralicek AV, Newcomb RD. Insights into subunit interactions within the insect olfactory receptor complex using FRET. Insect Biochem Mol Biol. 2013;43:138–45.
Sims C, Withall DM, Oldham N, Stockman R, Birkett M. Computational investigation of aphid odorant receptor structure and binding function. J Biomol Struct Dyn. 2023;41:3647–58.
Carraher C, Dalziel J, Jordan MD, Christie DL, Newcomb RD, Kralicek AV. Towards an understanding of the structural basis for insect olfaction by odorant receptors. Insect Biochem Mol Biol. 2015;66:31–41.
Bobkov YV, Walker IW, Cattaneo AM. Altered functional properties of the codling moth orco mutagenized in the intracellular loop-3. Sci Rep. 2021;11:3893.
Pacalon J, Audic G, Magnat J, Philip M, Golebiowski J, Moreau CJ, et al. Elucidation of the structural basis for ligand binding and translocation in conserved insect odorant receptor co-receptors. Nat Commun. 2023;14:8182.
Butterwick JA, Del MJ, Kim KH, Kahlson MA, Rogow JA, Walz T, et al. Cryo-EM structure of the insect olfactory receptor orco. Nature. 2018;560:447–52.
Zhao J, Chen AQ, Ryu J, Del MJ. Structural basis of odor sensing by insect heteromeric odorant receptors. Science. 2024;384:1460–7.
Del MJ, Yedlin MA, Ruta V. The structural basis of odorant recognition in insect olfactory receptors. Nature. 2021;597:126–31.
Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–87.
Nakamura T, Gold GH. A cyclic nucleotide-gated conductance in olfactory receptor cilia. Nature. 1987;325:442–4.
Nordstrom KJ, Sallman AM, Edstam MM, Fredriksson R, Schioth HB. Independent HHsearch, Needleman–Wunsch-based, and motif analyses reveal the overall hierarchy for most of the G protein-coupled receptor families. Mol Biol Evol. 2011;28:2471–80.
Wilson RI. Early olfactory processing in Drosophila: mechanisms and principles. Annu Rev Neurosci. 2013;36:217–41.
Nakagawa T, Vosshall LB. Controversy and consensus: noncanonical signaling mechanisms in the insect olfactory system. Curr Opin Neurobiol. 2009;19:284–92.
Jones PL, Pask GM, Rinker DC, Zwiebel LJ. Functional agonism of insect odorant receptor ion channels. Proc Natl Acad Sci USA. 2011;108:8821–5.
Yang L, Demares F, Norris EJ, Jiang S, Bernier UR, Bloomquist JR. Bioactivities and modes of action of vuaa1. Pest Manag Sci. 2021;77:3685–92.
Sun H, Liu F, Ye Z, Baker A, Zwiebel LJ. Mutagenesis of the orco odorant receptor co-receptor impairs olfactory function in the malaria vector Anopheles coluzzii. Insect Biochem Mol Biol. 2020;127:103497.
Wang Y, He X, Qiao L, Yu Z, Chen B, He Z. CRISPR/Cas9 mediates efficient site-specific mutagenesis of the odorant receptor co-receptor (orco) in the malaria vector Anopheles sinensis. Pest Manag Sci. 2022;78:3294–304.
Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–14.
Rutzler M, Lu T, Zwiebel LJ. Galpha encoding gene family of the malaria vector mosquito Anopheles gambiae: expression analysis and immunolocalization of agalphaq and agalphao in female antennae. J Comp Neurol. 2006;499:533–45.
Boto T, Gomez-Diaz C, Alcorta E. Expression analysis of the 3 g-protein subunits, galpha, gbeta, and ggamma, in the olfactory receptor organs of adult Drosophila melanogaster. Chem Senses. 2010;35:183–93.
Deng Y, Zhang W, Farhat K, Oberland S, Gisselmann G, Neuhaus EM. The stimulatory galpha(s) protein is involved in olfactory signal transduction in Drosophila. PLoS ONE. 2011;6:e18605.
Sargsyan V, Getahun MN, Llanos SL, Olsson SB, Hansson BS, Wicher D. Phosphorylation via PKC regulates the function of the Drosophila odorant co-receptor. Front Cell Neurosci. 2011;5:5.
Getahun MN, Thoma M, Lavista-Llanos S, Keesey I, Fandino RA, Knaden M, et al. Intracellular regulation of the insect chemoreceptor complex impacts odour localization in flying insects. J Exp Biol. 2016;219:3428–38.
Jain K, Lavista-Llanos S, Grabe V, Hansson BS, Wicher D. Calmodulin regulates the olfactory performance in Drosophila melanogaster. Sci Rep. 2021;11:3747.
Afify A, Betz JF, Riabinina O, Lahondere C, Potter CJ. Commonly used insect repellents hide human odors from Anopheles mosquitoes. Curr Biol. 2019;29:3669–80.
Dennis EJ, Goldman OV, Vosshall LB. Aedes aegypti mosquitoes use their legs to sense DEET on contact. Curr Biol. 2019;29:1551–6.
Matthews BJ, Dudchenko O, Kingan SB, Koren S, Antoshechkin I, Crawford JE, et al. Improved reference genome of Aedes aegypti informs arbovirus vector control. Nature. 2018;563:501–7.
Yan R, Chen P, Xu Z, Qian J, Zhu G, Jin Y, et al. A potential link between aromatics-induced oviposition repellency behaviors and specific odorant receptor of Aedes albopictus. Pest Manag Sci. 2024;80:3603–11.
Dong K, Du Y, Rinkevich F, Nomura Y, Xu P, Wang L, et al. Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochem Mol Biol. 2014;50:1–17.
Valbon W, Andreazza F, Oliveira EE, Liu F, Feng B, Hall M, et al. Bioallethrin activates specific olfactory sensory neurons and elicits spatial repellency in Aedes aegypti. Pest Manag Sci. 2022;78:438–45.
Grant GG, Estrera RR, Pathak N, Hall CD, Tsikolia M, Linthicum KJ, et al. Interactions of deet and novel repellents with mosquito odorant receptors. J Med Entomol. 2020;57:1032–40.
Dekel A, Sar-Shalom E, Vainer Y, Yakir E, Bohbot JD. The ovipositor cue indole inhibits animal host attraction in Aedes aegypti (Diptera: Culicidae) mosquitoes. Parasit Vectors. 2022;15:422.
Xu P, Choo YM, Chen Z, Zeng F, Tan K, Chen TY, et al. Odorant inhibition in mosquito olfaction. Iscience. 2019;19:25–38.
Xu P, Choo YM, An S, Leal GM, Leal WS. Mosquito odorant receptor sensitive to natural spatial repellents and inhibitory compounds. Insect Biochem Mol Biol. 2022;144:103763.