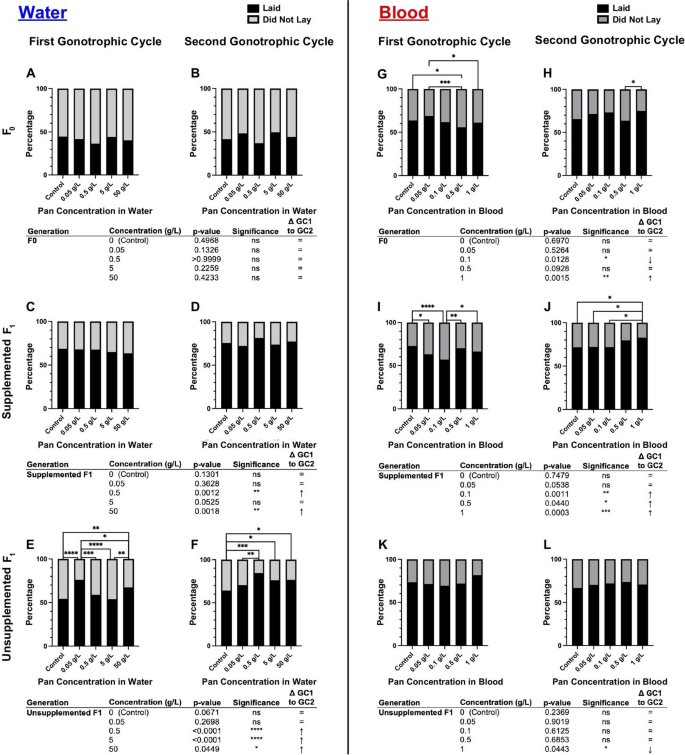
Pan effects on blood feeding tendency differed based on supplementation scheme, with transgenerational effects for both water and blood delivery
Pan supplementation via water had no effect on the tendency to consume a blood meal in F0 mosquitoes (Fig. 3A, B; Supplementary Material Table S1). In F1 offspring, effects on blood feeding tendency varied with concentration. Specifically, supplemented F1 provisioned with Pan exhibited significant differences in their tendency to take a first blood meal (Fig. 3C), with differences between treatment groups varying by concentration. There were, however, no significant effects on supplemented F1 tendency to take a second blood meal (Fig. 3D). Unsupplemented F1 offspring of F0 mosquitoes supplemented with 50 g/L Pan were significantly more likely to take a first blood meal compared with all other groups (Fig. 3E), but there were no effects on tendency to take a second blood meal (Fig. 3F).
Pan effects on blood feeding tendency differed on the basis of the supplementation scheme, with transgenerational effects for both water and blood delivery. Left (A–F): percentages of females that fed or did not feed following 3 days of Pan supplementation via water. Right (G–L): percentages of females that fed or did not feed following Pan supplementation via blood. Results are shown for the first blood meal (A,C,E,G,I,K) and second blood meal (B,D,F,H,J,L). Tables show comparisons of blood feeding tendency between first and second blood meals within the same treatment group. Water supplementation: F0 N = 6 replicates, supplemented F1 N = 4 replicates, unsupplemented F1 N = 3 replicates. Blood supplementation: F0 first blood feed = 5 replicates, F0 second blood feed = 4 replicates, supplemented F1 = 4 replicates, unsupplemented F1 = 3 replicates. Data were analyzed by chi-squared test (α = 0.05). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
Pan provisioning via blood meal was associated with blood feeding patterns that were distinct from those associated with provisioning by water. In contrast to Pan supplementation in water, F0 females provisioned with Pan via blood meal exhibited concentration-dependent differences in tendency to take first and second blood meals (compare Fig. 3A, B with Fig. 3G, H; Supplementary Material Table S1), with increased tendency to take a second blood meal in females supplemented with 0.05 or 1 g/L Pan relative to controls (Fig. 3H). Notably, F0 females that were provisioned with 0.1 g/L Pan were less likely to take first and second blood meals compared with other treatment groups (Fig. 3G,H). Additionally, in contrast to Pan supplementation in water, there were no differences among supplemented F1 offspring in tendency to take first or second blood meals (compare Fig. 3C,D with Fig. 3I,J). However, unsupplemented F1 offspring from both water-supplemented mothers (Fig. 3E,F) and blood-supplemented mothers (Fig. 3K,L) exhibited differences in tendency to take a first blood meal, but not a second blood meal, with F1 offspring of females supplemented with 0.5 g/L less likely to feed than all other groups (Fig. 3K). Notably, these results revealed transgenerational effects of Pan supplementation via both water and blood in that both sets of unsupplemented F1 offspring from supplemented mothers exhibited differences across groups in tendency to take a first blood meal (Fig. 3E,K).
To explore these differences further, we compared feeding tendency within a single treatment and filial group across first and second blood meals (e.g., control F0 tendency to take a first blood meal versus a second blood meal). When Pan was provisioned via water (Fig. 3A–F), significant differences within the F0 group and within each F1 group were associated with higher and lower feeding tendencies by treatment between the first and second blood meals. Notably, we saw a significant decrease in tendency to take a second blood meal compared with a first in both supplemented and unsupplemented F1 offspring of mothers provisioned with 50 g/L Pan (Fig. 3C–F). When Pan was provisioned via blood meal (Fig. 3G–L), F0 females that received Pan exhibited no difference in feeding tendency, whereas controls were less likely to take a second blood meal. Significant differences in supplemented F1 offspring were associated with increased feeding in the second versus first blood meals (Fig. 3I and J), while unsupplemented F1s from mother supplemented with 0.1 g/L Pan exhibited reduced feeding in the second versus first blood meal (Fig. 3K and L).
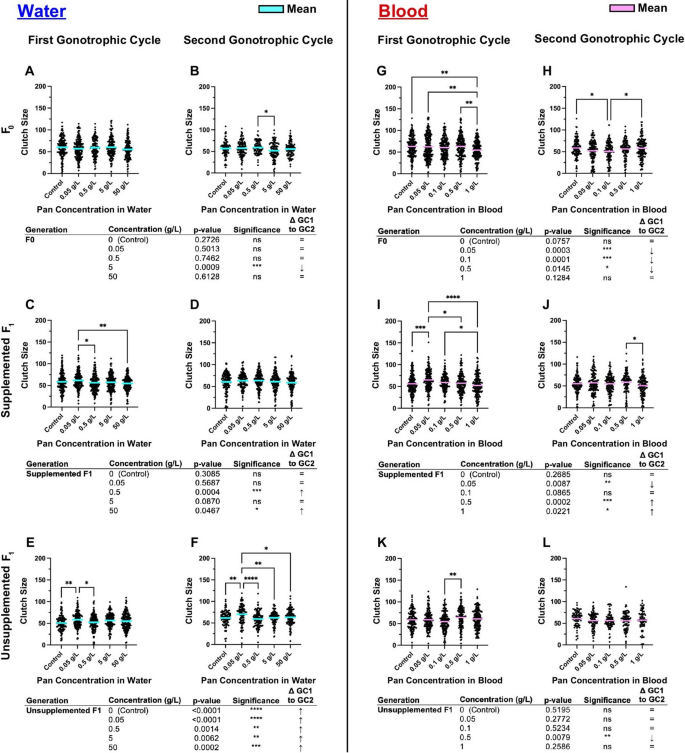
Pan effects on oviposition differed on the basis of supplementation scheme, with transgenerational effects only for water delivery and within generation effects only for blood delivery
Our previous work showed that pantazine provisioning to young (3–5-day-old) A. stephensi decreased Pan and increased CoA levels [6], but there were no effects on egg production in these young females [13]. In subsequent work, however, we showed that Pan is allocated to A. stephensi ovaries as eggs develop, that knockdown of the putative A. stephensi Pan transporter significantly reduced follicle development, and that Pan supplementation in blood restored the reproductive output of 14-day-old A. stephensi [14]. Together, these data suggested that Pan is an important and potentially limiting nutrient for mosquito reproduction.
To assess the impacts of Pan supplementation on oviposition, we individually housed bloodfed F0 mosquitoes and their supplemented or unsupplemented F1 offspring to determine egg production per female. Females completed two reproductive cycles following provisioning of Pan via water or blood, as previously described.
When Pan was supplemented via water (Fig. 4A–F; Supplementary Material Table S2), effects on oviposition were limited to unsupplemented F1 offspring (Fig. 4E,F), which were equally or more likely to oviposit across the first (GC1) and second (GC2) gonotrophic cycles relative to F1s from control mothers (Fig. 4E,F). By contrast, when Pan was supplemented via blood (Fig. 4G–L; Supplementary Material Table S2), significant effects on oviposition were observed in both F0s (Fig. 4G,H) and supplemented F1 offspring (Fig. 4I,J) but not in unsupplemented F1 offspring (Fig. 4K,L). In F0 females (Fig. 4G,H), these effects varied with treatment and gonotrophic cycle. In supplemented F1 offspring, however, females supplemented with 0.05 g/L and 0.1 g/L Pan were significantly less likely to oviposit than controls in GC1 (Fig. 4I). In GC2, however, F1 offspring supplemented with 1 g/L Pan were more likely than other groups to oviposit (Fig. 4J).
Pan effects on oviposition differ with supplementation method. Left (A–F): percentages of females that laid or did not lay eggs following 3 days of Pan supplementation via water and an unsupplemented blood meal. Right (G–L): percentages of females that laid or did not lay eggs following a Pan-supplemented blood meal. Results are shown for the first gonotrophic cycle (GC1; A,C,E,G,I,K) and second gonotrophic cycle (GC2; B,D,F,H,J,L). Tables show comparisons of oviposition percentages between GC1 and GC2 within the same treatment group. Water supplementation: F0 N = 6 replicates, supplemented F1 N = 4 replicates, unsupplemented F1 N = 3 replicates. Blood supplementation: F0 GC1 = 5 replicates, F0 GC2 = 4 replicates, supplemented F1 = 4 replicates, unsupplemented F1 = 3 replicates. Data were analyzed by chi-squared test (α = 0.05). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
To explore these differences further, we compared oviposition within a single treatment and filial group across GC1 and GC2 (e.g., control F0 oviposition in GC1 versus GC2). In A. stephensi supplemented with Pan via water (Fig. 4A-F), no F0 treatment groups exhibited differences in oviposition between GC1 and GC2 (Figs. 4A and B). However, both supplemented and unsupplemented F1s of F0s that received higher concentrations of Pan in water showed increased oviposition in GC2 compared with GC1 (Fig. 4C–F). In females supplemented with Pan via blood meal (Figs. 4G–L), effects on F0 groups were variable with two groups showing decreased and increased oviposition relative to control (Fig. 4G and H). Supplemented F1 offspring, however, were significantly more likely to oviposit in GC2 compared with GC1 for the three highest concentrations of Pan supplementation (Fig. 4I and J), whereas effects on unsupplemented F1 offspring were limited to decreased oviposition in GC1 versus GC2 at the highest Pan dose provided to F0 mothers (Fig. 4K and L).
Taken together, when Pan was provisioned in water, only unsupplemented F1 offspring showed increased oviposition in GC1 and GC2 compared with control (Fig. 4E,F). These data suggest that F0 supplementation with Pan in water is associated only with transgenerational effects on A. stephensi reproduction. By contrast, Pan provisioning via blood meal was associated with effects on oviposition only during supplementation (Fig. 4G–J), with no transgenerational effects on unsupplemented F1 offspring (Fig. 4K,L).
Pan supplementation altered clutch sizes within generations and transgenerationally, with universal increases between GC1 and GC2 in unsupplemented F1s from water-supplemented mothers
When Pan was supplemented via water (Fig. 5A–F; Supplementary Material Tables S3,S4), effects were noted in supplemented mothers in GC2 (Fig. 5B), in supplemented F1 offspring in GC1 (Fig. 5C) and unsupplemented F1 offspring in GC1 and GC2 (Fig. 5E,F). In particular, the supplemented and unsupplemented offspring of mothers supplemented with 0.05 g/L Pan had the greatest mean clutch sizes in these cycles. Increased oviposition in unsupplemented F1 offspring from mothers supplemented with 0.05 g/L Pan (Fig. 4E) was also consistent with larger clutch sizes in this group in both GC1 and GC2 (Fig. 5E,F).
Pan supplementation altered clutch sizes within generations and transgenerationally based on method. Left (A–F): the number of eggs per female following 3 days of Pan supplementation via water and an unsupplemented blood meal. Right (G–L): the number of eggs per female following a Pan supplemented blood meal. Results are shown for the first gonotrophic cycle (GC1; A,C,E,G,I,K) and second gonotrophic cycles (GC2; B,D,F,H,J,L). Tables show comparisons of clutch size between GC1 and GC2 within the same treatment group. Water supplementation: F0 N = 6 replicates, supplemented F1 N = 4 replicates, unsupplemented F1 N = 3 replicates. Blood supplementation: F0 GC1 = 5 replicates, F0 GC2 = 4 replicates, supplemented F1 = 4 replicates, unsupplemented F1 = 3 replicates. Data were analyzed by one-way ANOVA (α = 0.05). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
When Pan was supplemented via blood meal (Fig. 5G–L; Supplementary Material Tables S3,S4), significant effects were observed during supplementation of F0s (Fig. 5G,H) and supplemented F1 offspring (Figs. 5I,J), with a single difference in clutch size noted in unsupplemented F1 offspring in GC1 (Fig. 5K). The latter was reflective of the lack of differences in oviposition in GC1 and GC2 in this group (Fig. 4K,L). Within groups, F0s supplemented with 1 g/L Pan had a significantly smaller mean clutch size compared with control in GC1 (Fig. 5G), while in GC2, F0s supplemented with 0.1 g/L Pan had a significantly smaller mean clutch size than the control (Fig. 5H). In the offspring of blood-supplemented females, mean clutch size was highest in GC1 in F1 supplemented females provisioned with 0.05 g/L Pan (Fig. 5I), but this pattern was not observed in GC2 (Fig. 5J).
To explore these differences further, we compared clutch sizes within a single treatment and filial group across GC1 and GC2 (e.g., control F0 clutch size in GC1 versus GC2). When Pan was provisioned in water (Fig. 5A–F), a single decrease in F0 clutch size in GC1 versus GC2 was noted for one treatment group (Fig. 5A,B). This contrasted with increased clutch sizes in two treatment groups for supplemented F1s and increased clutch sizes for all unsupplemented F1 groups between GC1 and GC2 (Fig. 5C–F). When Pan was provisioned via blood meal (Fig. 5G–L), F0 clutch sizes were more frequently reduced by treatment compared with water supplementation (three F0 blood-supplemented groups in Fig. 5G,H versus one F0 water-supplemented group in Fig. 5A,B). Clutch size between GC1 and GC2 in blood-supplemented F1 offspring was decreased at the lowest level of Pan supplementation and increased at the two highest levels of supplementation (Fig. 5I, J). In contrast to universal increases in clutch sizes between GC1 and GC2 in unsupplemented F1 offspring of water-supplemented mothers (Fig. 5E,F), only a single treatment (0.5 g/L) delivered via blood meal to F0s was associated with a change in unsupplemented F1 clutch size, a decrease between GC1 and GC2 (Fig. 5K, L).
Pan supplementation minimally altered offspring sex ratio
In several vertebrate and invertebrate species, alterations to endogenous Pan levels have been shown to impact offspring sex [21, 25, 26]. To determine whether Pan supplementation altered sex ratio in A. stephensi, we counted female and male offspring from each of our treatment groups. Given the large sample sizes of males and females across our replicates, several significant differences were observed, but deviations from the expected 50:50 sex ratio were small (Fig. 6A–L; Supplementary Material Table S5).
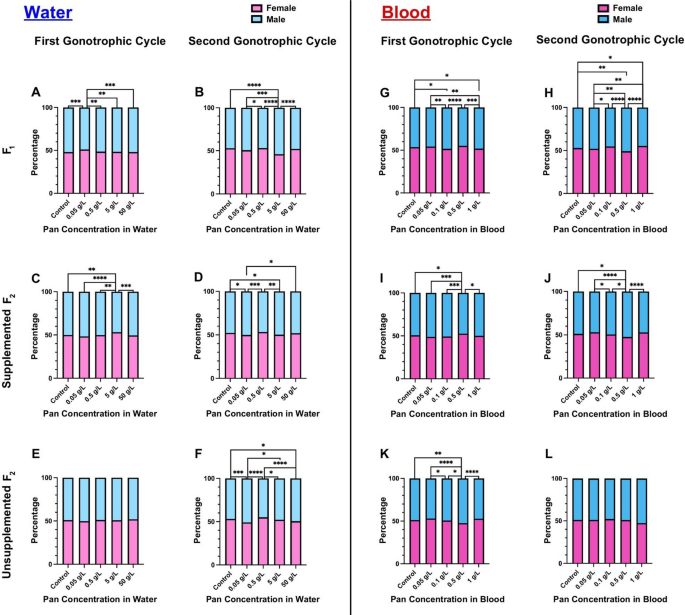
Pan supplementation minimally altered offspring sex ratio. Left (A–F): the percentages of female and male offspring following 3 days of Pan supplementation via water and an unsupplemented blood meal. Right (G–L): the percentages of male and female offspring following a Pan-supplemented blood meal. Results are shown for the first gonotrophic cycle (A,C,E,G,I,K) and second gonotrophic cycle (B,D,F,H,J,L). Water supplementation: F0 N = 6 replicates, supplemented F1 N = 4 replicates, unsupplemented F1 N = 3 replicates. Blood supplementation: F0 GC1 = 5 replicates, F0 GC2 = 4 replicates, supplemented F1 = 4 replicates, unsupplemented F1 = 3 replicates. Data were analyzed by chi-squared test (α = 0.05). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
Pan supplementation via water did not alter fecundity of 14-day-old females
We showed previously that Pan levels in female A. stephensi declined by more than 50% by 14 days post-adult eclosion [14]. Further, Pan provisioning via a blood meal reversed significant declines in oviposition and clutch size in 14-day-old A. stephensi females to levels observed in 3–5-day-old females [14]. In this study, Pan provisioning via water was associated with transgenerational effects, increasing oviposition and clutch size in unsupplemented F1 females (Figs. 4E,F and 5E,F). By GC2, females in these experiments were over 14 days old, suggesting that Pan provisioning of 14-day-old females via water might recover the loss in fecundity associated with aging or achieve increased fecundity similar to that observed in unsupplemented F1 offspring of water-supplemented mothers.
In contrast to our expectations, we observed no significant changes to oviposition or clutch size in water-supplemented 14-day-old females (Fig. 7A,B). Accordingly, the transgenerational effects of Pan provisioning to 3–5-day-old females that are 14 days old at the time of observed increases in fecundity cannot be replicated by provisioning Pan via water directly to 14-day-old females. As noted, delivery of Pan in blood to 14-day-old females recovered fecundity to levels observed in 3–5-day-old females [14]. Taken together, the effects of Pan supplementation observed in our current study are transgenerational at 14 days post-adult eclosion, while the previously observed effect of blood delivery of Pan to 14-day-old females occurred within the reproductive cycle of those supplemented females [14].
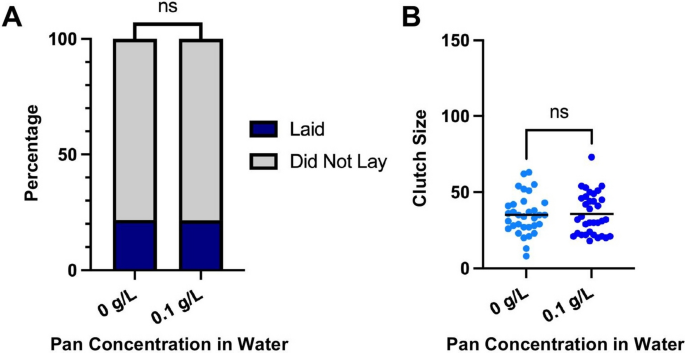
Pan supplementation via water did not alter fecundity of 14-day-old females. A The percentages of females that laid or did not lay. Data were analyzed by chi-squared test (α = 0.05). B Numbers of eggs laid per female. Data were analyzed by two-tailed t-test (α = 0.05)
Ovary Pan levels in unsupplemented F1 offspring from water-supplemented mothers reflected transgenerational effects on oviposition and clutch size
Given the association between F0 water supplementation and transgenerational effects on unsupplemented F1 oviposition (Fig. 4E,F) and clutch size (Fig. 5E,F), we sought to determine whether Pan levels in these unsupplemented offspring were reflective of the F0 supplementation scheme (Fig. 2). In combined data from replicated studies, there were no differences in Pan levels in whole bodies or carcasses of unsupplemented F1 offspring from control mothers or mothers supplemented in water or blood (Fig. 8A,B). However, Pan levels in ovaries from unsupplemented F1 offspring were significantly lower than ovary levels from F1 unsupplemented offspring derived from control mothers or mothers supplemented via blood (Fig. 8C). Further, ovary Pan levels in unsupplemented F1 offspring from blood-supplemented mothers did not differ from control levels (Fig. 8C), a pattern reflective of the lack of transgenerational effects on oviposition of unsupplemented F1 offspring from blood-supplemented mothers (Fig. 4K,L). Hence, altered ovary Pan levels were specifically associated with increased fecundity of unsupplemented F1 offspring from water-supplemented mothers.
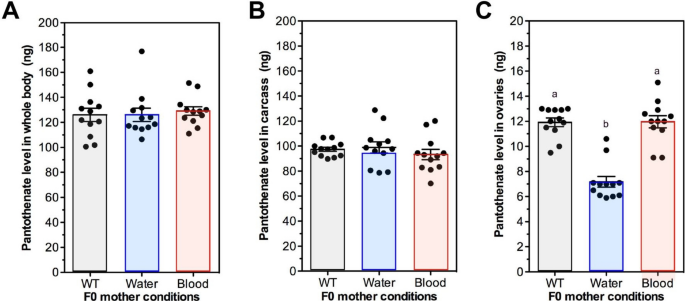
Ovary Pan levels in unsupplemented F1 offspring from water-supplemented mothers were reduced relative to levels in control (WT) and blood-supplemented mothers. Data were collected from tissues from two separate A. stephensi cohorts prepared as shown in Fig. 2. A Whole body Pan levels of unsupplemented F1 females derived from F0 mothers not supplemented with Pan (WT) or supplemented with 0.1 g/L Pan in water or in blood. B Carcass Pan levels in unsupplemented F1 females as in (A). (C) Ovary Pan levels in F1 unsupplemented F1 females as in (A). Data were analyzed by ANOVA and Tukey’s test (α = 0.05). Different lowercase letters above bars indicate significant differences between groups