These placebo-controlled laboratory comparative efficacy studies were conducted by ClinVet in Bloemfontein, South Africa (study 1), and New York, USA (study 2). All cats were under the care of a licensed veterinarian at all times, and studies complied with all applicable local laws, state laws, and national regulations related to the humane care and use of animals. All study protocols were approved by the Study Site Institutional Animal Care and Use Committee, and all study procedures were in accordance with the relevant World Association for the Advancement of Veterinary Parasitology guidelines [22,23,24].
Study design
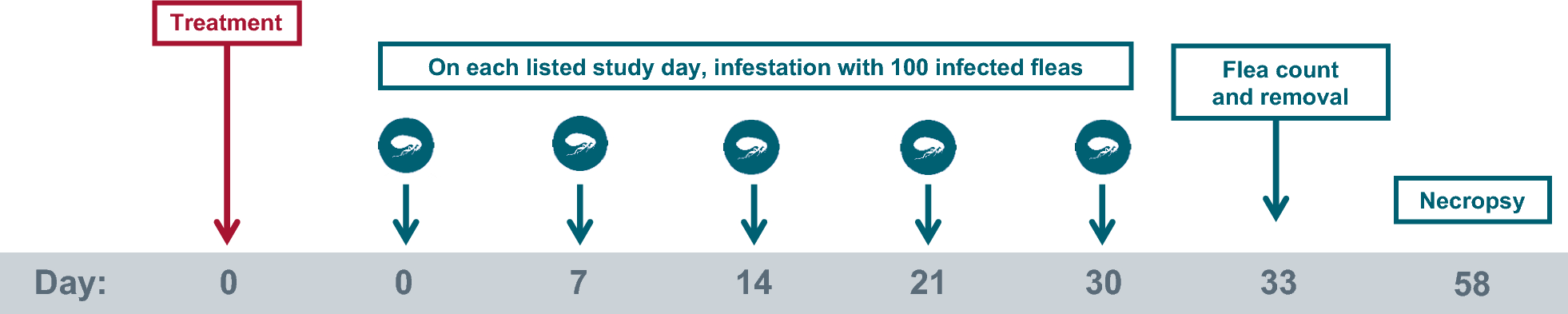
The study design used in both study 1 and 2 is provided in Fig. 1. The host suitability for C. felis was determined by infesting candidate cats (n = 24 per study) on Day −8 (study 1) or Day −21 (study 2) with C. felis (not infected with D. caninum). At 24 h post-infestation, cats were examined and comb-counted, and the 20 cats with the highest flea counts were selected for each study. Cats were allocated to treatments, rooms, and cages according to a randomized complete block design with blocking based on pre-infestation flea counts. Blocks consisted of two cats, with one cat randomized to each treatment within the block. Cats in the same block were randomized to cages located near each other within each room. Enrolled cats were moved to their allocated cages within 8 days of study start and treated on Day 0 with either placebo or with Revolution Plus. All cats were infested with 100 (± 5) viable, unfed, adult C. felis fleas on Days 0, 7, 14, 21, and 30. Cats were allowed to engage in normal grooming behavior throughout the course of the study to mimic the natural infection method of D. caninum. Live flea counts were conducted on Day 33, with removal of the fleas. All cats were euthanized on Day 58, and necropsies were performed to recover, identify, and count D. caninum scolices from the gastrointestinal tracts of each cat. Masking was accomplished by the separation of the functions of study personnel. All persons making observations, performing polymerase chain reaction (PCR) testing; conducting scolex counts, post-treatment flea infestations, and post-treatment flea counts; or performing general care for the cats were masked to experimental treatments.
Study design used in study 1 and 2
Animals
All cats enrolled in these two studies were healthy and clinically normal as determined by a veterinarian with experience in the practice of general feline medicine. All enrolled cats were confirmed negative for D. caninum infections based on PCR analysis of fecal samples [25] within 14 days of study start on Day 0. Study 1 utilized 12 males and 8 females aged 16–113 months and weighing 2.8–4.7 kg on Day −1. Study 2 utilized ten males and ten females aged 8–47 months of age and weighing 2.7–7.7 kg on Day −1. Cats were intact or neutered, none were pregnant or lactating, and all were uniquely identified. All cats had been regularly dewormed and previously vaccinated as required, but no cat was given medication from Day −14 (study 1) or Day −21 (study 2) through study end (unless concomitant treatment was required to maintain adequate care). All cats were housed in individual, walled cages such that no physical contact between cats or transmission of fleas was possible, and cats were not moved between cages after Day 0. Cats were fed a commercial dry food once per day, and fresh water was provided ad libitum. General health observations were performed daily for each cat starting on Day −14 (study 1) or Day −21 (study 2) through the duration of the study.
Flea infestations and assessment
Study 1 used viable, unfed adult C. felis fleas from a colony originally sourced from PLRS laboratories (Corapeake, North Carolina, USA) in 2010 and enriched with fleas from Sierra Research Laboratories (Modesto, CA, USA) in 2017. Study 2 used viable, unfed adult C. felis originally sourced from Ecto Services, Inc. (Henderson, North Carolina, USA) in 2020 and enriched with fleas from Henderson, North Carolina, in 2022. All fleas were infected for the purpose of the studies with D. caninum, collected from cats in the field either in Thessaloniki, Greece, in 2019 (study 1) or in Oklahoma, USA, in 2015 (study 2), as follows: freshly collected flea eggs were added to D. caninum proglottids for a period of 3 days. After the flea eggs hatch, the resulting larvae are exposed to the proglottids and are thereby forced to feed on them. These flea larvae were then transferred to a standard flea breeding medium, where they were allowed to complete their developmental cycle. The fleas were confirmed to be infected with D. caninum at an infection rate ranging from 23.0% to 53.3% in study 1 and from 6.8% to 20.0% in study 2. Both in study 1 and 2, a total of 24 cats were infested with 100 (± 5) viable, unfed adult (and D. caninum-free) C. felis fleas to determine host suitability. Then, 1 day after infestation, the number of live fleas present on each cat was counted, and fleas were removed, and the 20 cats with the highest flea counts were randomly allocated to one of two treatment groups blocked on flea counts. After treatment, cats were infested with 100 (± 5) viable, unfed adult and infected fleas on Days 0, 7, 14, 21, and 30. During infestations, cats remained in their respective cages. A vial containing 100 (± 5) fleas was swirled to form a flea pellet, which was then deposited onto the cat’s hair coat as close as possible to the skin at a site distal to the site of treatment. The vial was held in position for ~10 s to ensure the flea pellet was not dislodged and to facilitate dispersal of the fleas into the hair coat.
Live flea counts with removal of the fleas were conducted on Day 33, 72 (± 2) h after Day 30 infestation. Each cat was removed from its cage and placed upon a disinfected table. Fine-toothed flea combs were used to comb the entire body of the cat, starting with the anterior and working toward the posterior end and ventral areas. Any collected fur was removed from the comb and separated, and fleas within the fur were counted. Cats were combed for at least 10 min. Personnel conducting counts were masked to treatment assignments and changed gloves between each cat.
All cats were euthanized on Day 58. The gastrointestinal tracts were removed and ligated to separate the stomach, small intestines, large intestines, and the end of the rectum. Each section was cut open, and the mucosa was observed macroscopically for the presence of worms, which were collected and preserved. The mucosa was washed with water and scraped, with the washings and scrapings collected separately, washed through 0.150 mm sieves. The collected material was examined using a stereomicroscope, and the number of scolices were counted and recorded.
Treatment and observations
Clinical observations and administration site evaluations were performed on all cats prior to treatment on Day 0. All cats were then treated with either placebo (Revolution Plus formulation without the active ingredients) or with Revolution Plus at its minimum recommended dose (6.0 mg/kg selamectin, 1.0 mg/kg sarolaner). Doses were calculated using body weights recorded on Day −1. The entire dose was administered to the skin at a single site, at the base of the neck cranial to the scapulae. The site of administration was evaluated at 1 h (± 15 min), 3 h (± 30 min), 6 (± 1) h, and 24 (± 1) h after treatment as well as on Days 3, 5, and 58 for cosmetic changes (matting, spiking/stiff hair, wetness, white deposits), alopecia, erythema, and edema. Clinical observations were completed at 1 h (± 15 min), 3 h (± 30 min), 6 (± 1) h, and 24 (± 1) h after treatment.
Statistical analyses
Adequate challenge was defined as 60% of the placebo-treated control cats maintaining ≥ 50 fleas following the final flea infestation on Day 30 and 60% of the placebo-treated control cats having ≥ 2 adult D. caninum at the time of necropsy. The experimental unit was the individual cat. Scolex counts and flea counts were analyzed with a general linear mixed model containing the fixed effect of treatment and the random effects of room. For both scolex and flea counts, the treatments were compared, and efficacy was calculated as follows: 100 × (control mean – revolution plus mean)/control mean. For flea counts, means, standard errors, 95% confidence limits, minimums, and maximums were calculated. Efficacy was calculated using the least squares means as estimates of the arithmetic means. Scolex counts were transformed with a natural logarithm transformation prior to analysis. Least squares means, standard errors, 95% confidence limits, minimums, and maximums were calculated. Efficacy was calculated using the geometric (back-transformed least squares) means.