According to the World Health Organization (WHO), Dengue is a viral infection spread to humans by infected mosquitoes and is a leading cause of febrile illness among international travelers.
Based on the record-setting number of Dengue outbreaks in 2024, the WHO has classified Dengue as a grade 3 emergency, placing about four billion people at risk globally.
In recent years, the development of effective vaccines to prevent Dengue has been an essential factor in the global effort to reduce outbreaks.
Still, strategies to maximize second-generation vaccine coverage in endemic regions are needed.
According to a new study published in the journal Vaccines (Volume 62, August 30, 2025, 127558), dengue-endemic countries, such as the Kingdom of Thailand, have already initiated national Human Papillomavirus (HPV) school-based vaccination programs based on recommendations from the WHO.
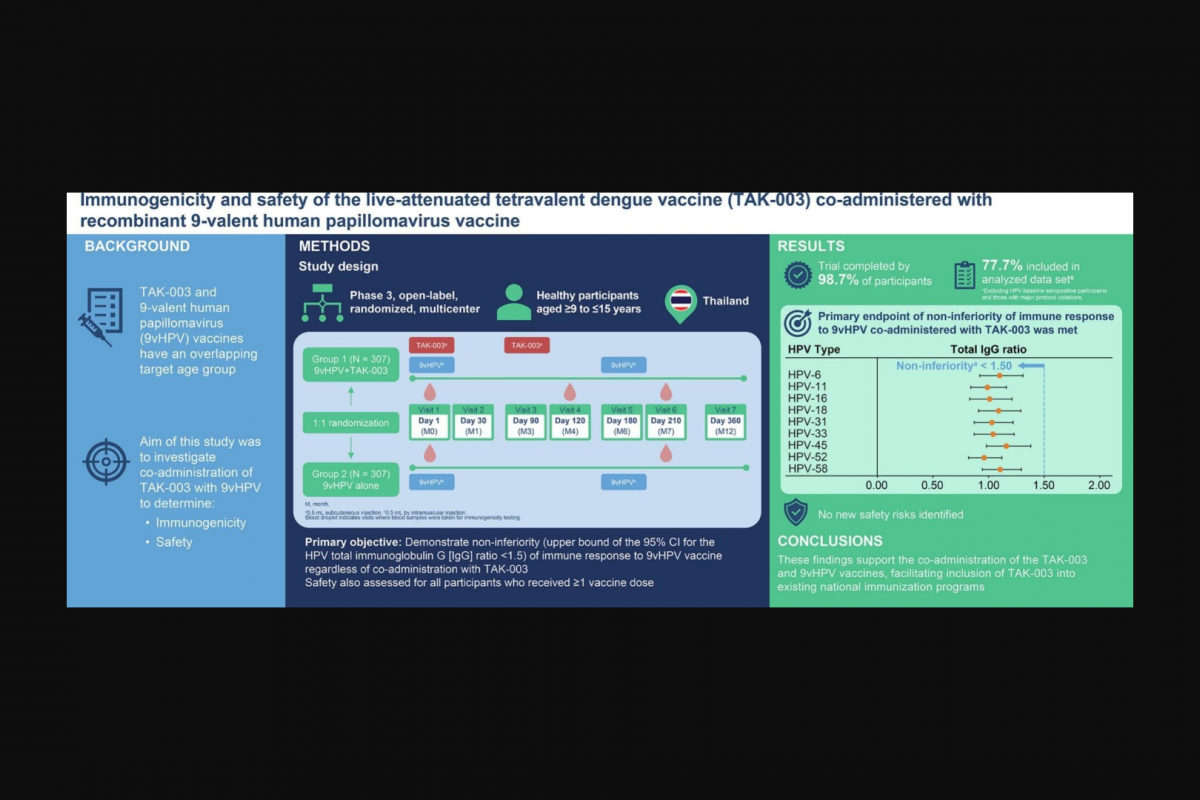
These researchers wrote, ‘Due to the overlapping age range targeted by many vaccines, including 9vHPV (Merck GARDASIL 9®) and TAK-003 (Takeda’s QDENGA®), integration of Dengue vaccination into these existing programs could be a beneficial approach to increase vaccine coverage and reduce operational costs.’
This phase 3, multicenter clinical trial (NCT04313244) demonstrated the non-inferiority (NI) of the immune response to 9vHPV when co-administered with the live-attenuated dengue vaccine, TAK-003, compared with 9vHPV administered alone, with the primary objective was met.
There was no evidence of interaction caused by the live-attenuated vaccine on the virus-like particle-based 9vHPV immunogenicity and safety.
Similarly, these data suggest that co-administration of TAK-003 with HPV does not compromise TAK-003 immunogenicity or safety.
In the co-administration group, an immune response was elicited against each of the four dengue serotypes, with GMTs of anti-dengue NAbs increasing to 494–1691 at M4 post-vaccination.
Participants who had a previous exposure to Dengue (Dengue seropositive at baseline) showed higher post-vaccination GMTs, which can be explained by the presence of immune memory from their prior dengue infection.
As observed in previous studies with TAK-003, almost all participants (99.6%) had tetravalent seropositivity at M4, irrespective of baseline serostatus.
The co-administration of 9vHPV and TAK-003 was well-tolerated, and there was no clinically relevant adverse impact on the safety or reactogenicity, with no AEs leading to vaccine withdrawal or trial discontinuation.
A limitation of this trial is that a TAK-003-only arm was not included, meaning the NI of the TAK-003 immune response could not be directly evaluated after co-administration. One of the reasons behind this design was that the trial was only considered feasible in dengue-endemic regions.
As of August 4, 2025, Thailand’s Food and Drug Administration has approved the QDENGA vaccine and over 30 other countries, but not the United States. Currently, the U.S. is evaluating the first-generation Dengue vaccine (Dengvaxia) in children in Puerto Rico.
Note: This phase 3 clinical trial was sponsored by Takeda Vaccines, Inc., Cambridge, MA, USA, the producer of the QDENGA vaccine.