Since its development in 1926, MST has served as a critical diagnostic tool for cutaneous leishmaniasis (CL) globally, particularly for evaluating DTH to Leishmania infection [25]. It remains widely used in Latin America, where the principal etiological agents of ACL belong to the L. (Viannia) subgenus, including L. (V.) braziliensis, L. (V.) peruviana, L. (V.) panamensis, and L. (V.) guyanensis, which are well-recognized for their ability to stimulate a CD4⁺/Th1-type immune response and thereby elicit DTH reactions [26,27,28,29]. Recently, MST has been employed not only for its original diagnostic purpose but also for epidemiological studies as a screening tool for DTH responses associated with asymptomatic infections caused by L. (L.) infantum and L. (L.) donovani in regions endemic for visceral leishmaniasis. These applications further support the ability of MST as a reliable method for assessing DTH in human Leishmania infections [30,31,32,33,34,35,36,37,38,39,40,41].
By contrast, early studies into the use of MST in cases of ACL caused by L. (L.) amazonensis or L. (L.) pifanoi, particularly in ADCL form, were conducted several decades ago in Brazil [42, 43], Bolivia [44], and Venezuela [45]. These studies consistently reported negative MST results in patients with ADCL, leading to the prevailing view that this clinical form is linked to a specific immunodeficiency in the host. As a result, the absence of DTH in ADCL has been largely attributed to the host’s inability to generate an effective T-cell-mediated immune response, rather than to any direct immunosuppressive action of the parasite itself [46].
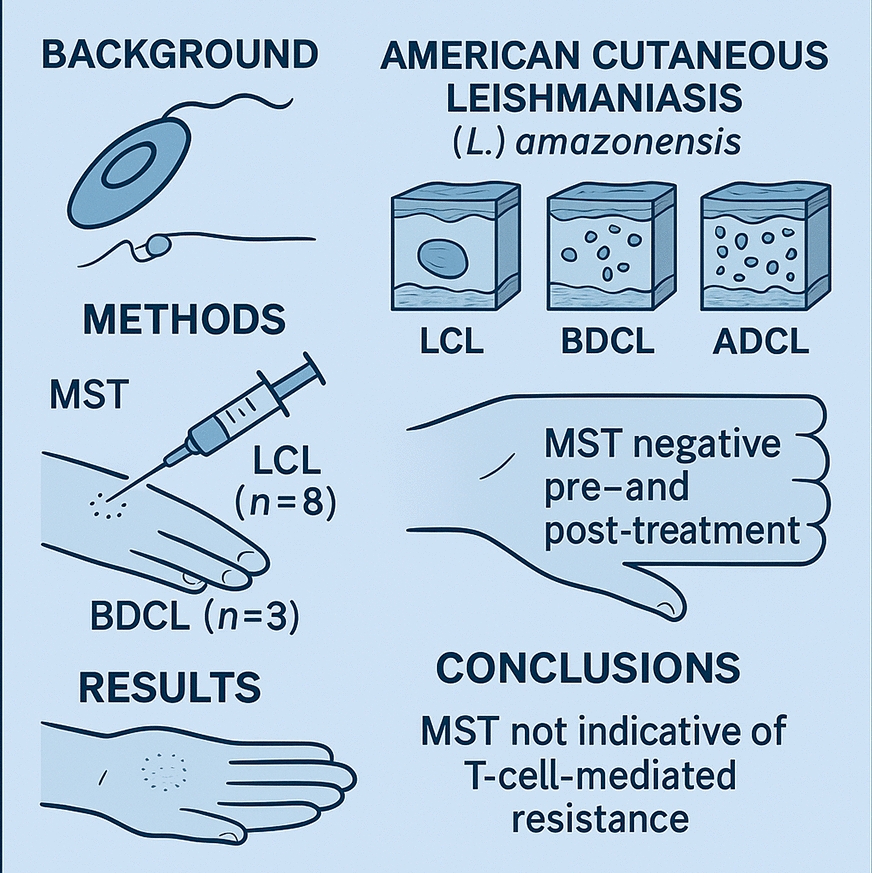
DTH suppression due to L. (L.) amazonensis infection was first detected in Brazil during the 1980s through a pioneering study conducted by our research group in Pará State. This study was the first to demonstrate that 57.7% of patients with LCL caused by L. (L.) amazonensis showed no MST reactivity when using homologous promastigote antigen [13]. Subsequent studies confirmed comparable or even higher rates of DTH suppression, ranging from 48.6% to 100% among patients with LCL, thereby establishing the absence of MST reactivity as a hallmark of L. (L.) amazonensis infection across the clinical–immunopathological spectrum of ACL. This phenomenon was most pronounced in ADCL, where MST reactivity was universally absent (100%), but was also observed to varying degrees in patients with LCL (48.6–100%), despite their generally favorable response to antimony therapy [8, 9, 14,15,16].
These findings of DTH suppression in L. (L.) amazonensis-induced LCL is further supported by histopathological findings. Skin lesions typically exhibit a dermal cellular infiltrate that is predominantly macrophagic, with scarce plasma cells and lymphocytes, and a notably rare presence of epithelioid cells, i.e., macrophages that are activated through T-cell-mediated immune response. The macrophages appear vacuolated and heavily parasitized, providing additional confirmation of impaired DTH expression at the site of infection [47]. This histological profile contrasts sharply with that observed in L. (Viannia) braziliensis-induced LCL, where the dermal infiltrate is characterized by a prominent presence of epithelioid cells, corresponding with a high prevalence (≥ 90%) of MST reactivity [48].
The present results demonstrated a complete absence of MST reactivity in all patients analyzed, including those with LCL and BDCL, irrespective of lesion count, clinical presentation, or disease duration, both before and after successfully antimony therapy. Although the absence of MST reactivity in LCL caused by L. (L.) amazonensis has been previously documented [14], this is the first report of such a finding in BDCL. Notably, in contrast to L. (V.) braziliensis infection, in which MST reactivity persists over time, even following antimony therapy, due to continued antigenic stimulation [49, 50], infection with L. (L.) amazonensis in both LCL and BDCL appears to be completely cleared by therapy. This clearance likely prevents the sustained antigenic stimulation of the T-cell-mediated immune response, including DTH, as assessed by MST. These findings suggest a previously unrecognized immunological profile linked to L. (L.) amazonensis infection and highlight the distinct immune mechanisms involved in ACL pathogenesis.
Of particular note is the first documented case of LCL spontaneous cure due to L. (L.) amazonensis, observed 1 year after diagnosis. Remarkably, the patient exhibited no MST reactivity either before or after the spontaneous resolution of the lesion, an observation that challenges current understanding of DTH in human Leishmania infection [26,27,28,29, 51]. Typically, spontaneous cure is associated with the activation of the T-cell-mediated immune response. However, this case suggests that L. (L.) amazonensis infection may inhibit the activation of genetic pathways essential for DTH expression, most likely linked to the CD4⁺/Th1-type immune response, thereby compromising macrophage-mediated resistance to infection [21, 52].
These findings raise a critical question: why do patients with L. (L.) amazonensis-induced LCL and BDCL, despite achieving clinical cure, whether through antimony therapy or spontaneously, fail to develop DTH, assessed by MST reactivity? This contrasts with patients affected by so-called Old and New World visceral leishmaniasis, who, despite profound suppression of the CD4⁺/Th1-type immune response, often exhibit a significant shift toward DTH, assessed by MST reactivity following treatment [53, 54]. A plausible explanation lies in the persistence of low-level infection. In L. (V.) braziliensis-induced LCL, for instance, antimony therapy does not fully eradicate the parasite from healed lesions, allowing ongoing antigenic stimulation and sustained DTH responses [49, 50]. Similarly, in treated or asymptomatic visceral leishmaniasis, incomplete parasite clearance, particularly in the liver, may promote posttreatment DTH conversion through the gradual reactivation of CD4⁺/Th1-type immune response [53,54,55,56]. By contrast, L. (L.) amazonensis infection appears to be entirely eliminated by treatment, potentially precluding such immunological reactivation.
In this case, DTH suppression in L. (L.) amazonensis infection, both in LCL and BDCL, appears to be influenced by two distinct parasite–host interaction mechanisms. The first involves the potential inhibition of genetic pathways that regulate DTH during the active phase of infection [21, 52]. The second, which may represent a novel observation, is associated with the absence of antigenic stimulation following the complete clearance of the parasite through antimony therapy. These findings underscore the critical role of species-specific host–parasite interactions in modulating the T-cell-mediated immune response, particularly the CD4⁺/Th1 and CD4⁺/Th2 pathways, which in turn govern DTH activation [3, 8, 9].
Supporting this hypothesis, it is noteworthy that, based on more than 40 years of clinical experience monitoring patients infected with L. (L.) amazonensis in Pará, Brazilian Amazon, this research group has not observed any cases of relapse following medium- or long-term cure with antimony therapy, particularly among patients with LCL. This observation stands in contrast to the more frequent relapses seen in LCL and BDCL cases due to L. (V.) braziliensis or L. (V.) guyanensis [Silveira, personal observation].
The progression of L. (L.) amazonensis infection appears to be influenced by the extent of DTH suppression, mainly due to the impairment of the CD4⁺/Th1-type immune response, in conjunction with host-specific factors such as age and genetic background. This may lead to the development of BDCL, an intermediate-severity form that predominantly affects young adults and typically requires twice the standard dosage of antimony therapy used for LCL [8, 9, 17]. In the absence of effective immune control, the infection may progress to ADCL, a severe and incurable form resistant to all types of chemotherapy. ADCL primarily affects children under 10 years of age and adults belonging to ethnic groups with heightened susceptibility to L. (L.) amazonensis infection [2, 10]. This clinical form is most frequently observed among historically enslaved individuals of African descent in the pre-Amazon region of Maranhão State, which reports the highest number of ADCL cases in Brazil [57].
In the majority of infected individuals, particularly those with LCL, accounting for ≥ 98% of cases, the parasite’s suppressive effect on the CD4⁺/Th1-type immune response, reflected by the absence of DTH in negative MST, appears to be less pronounced than in patients with BDCL and patients with ADCL. This relative preservation of immune function may be alternatively attributed to the immunomodulatory activity of CD8⁺ T cells, which exhibit significantly higher densities (P < 0.05) than CD4⁺ T cells across the clinical–immunopathological spectrum of ACL due to L. (L.) amazonensis, even after antimony therapy [12, 58]. These findings highlight the pivotal role of CD8⁺ T cells as a key resistance mechanism in the control of L. (L.) amazonensis infection, contributing to protective immunity by producing IFN-γ and promoting Th1 responses not only in human infection [8, 9, 12] but also in murine models [59, 60].
Leishmania (L.) amazonensis primarily modulates the human immune response by disrupting the balance between cellular and humoral immunity. Specifically, it suppresses the CD4⁺/Th1-type immune response, including DTH, which is essential for macrophage activation and effective parasite clearance, while enhancing the less effective, IgG-mediated humoral response [61, 62]. This immunomodulatory effect is particularly pronounced in BDCL and ADCL, which are characterized by high parasite loads [3, 8, 9, 63]. In the present study, patients with LCL exhibited low to moderate IgG titers (80–320), whereas those with BDCL showed significantly higher titers (640–2,560). These findings support the notion that L. (L.) amazonensis facilitates disease progression by impairing protective cellular immunity, primarily the CD4⁺/Th1-type response, while promoting a nonprotective humoral response.
Supporting these observations, a recent methodological advancement deserves attention: the optimization of MST using an L. (V.) lainsoni axenic amastigote antigen to assess DTH in ACL cases in the Brazilian Amazon. This approach yielded significantly higher mean reactivity (18.8 mm ± 13.3) in LCL caused by L. (Viannia) species, compared with the response elicited by the conventional L. (V.) braziliensis promastigote antigen (11.8 mm ± 8.2). Notably, the only patient in the study diagnosed with LCL due to L. (L.) amazonensis exhibited no reactivity to the MST, in contrast to 52 cases associated with L. (Viannia) species. This finding reinforces the suppression of DTH in L. (L.) amazonensis infections, even when using an antigen demonstrated to be more potent than the standard L. (V.) braziliensis antigen [64].
At the time of concluding this study, two individuals previously diagnosed with L. (L.) amazonensis-associated ACL were reevaluated. The first was a 58-year-old woman with ADCL, and the second was a 54-year-old man with BDCL. Both had undergone multiple treatment regimens, including antimonial compounds, pentamidine, and chemoimmunotherapy (BCG combined with Leishvacin), and they ultimately achieved clinical cure (Fig. 4, supplementary material) [17, 65]. Immunological testing revealed negative IFAT/enzyme-linked immunosorbent assay (ELISA)-IgG results for the woman and a negative MST for the man, confirming parasitological cure. Notably, the man’s prior MST conversion, documented 25 years earlier, was likely attributable to transient immunogenic stimulation induced by immunotherapy. These findings represent the first documented confirmation of parasitological cure in ADCL and BDCL caused by L. (L.) amazonensis, as evidenced by negative serological and DTH-based assays.
Finally, to underscore the complexity of the immune responses to L. (L.) amazonensis infection, it should be highlighted that an ADCL case with more than 30 years of disease evolution achieved clinical cure following a single intranasal dose of meglumine antimoniate, despite the patient remaining negative for the MST after cure [66]. Notably, this therapeutic approach was unsuccessful in four patients under our clinical supervision [67].