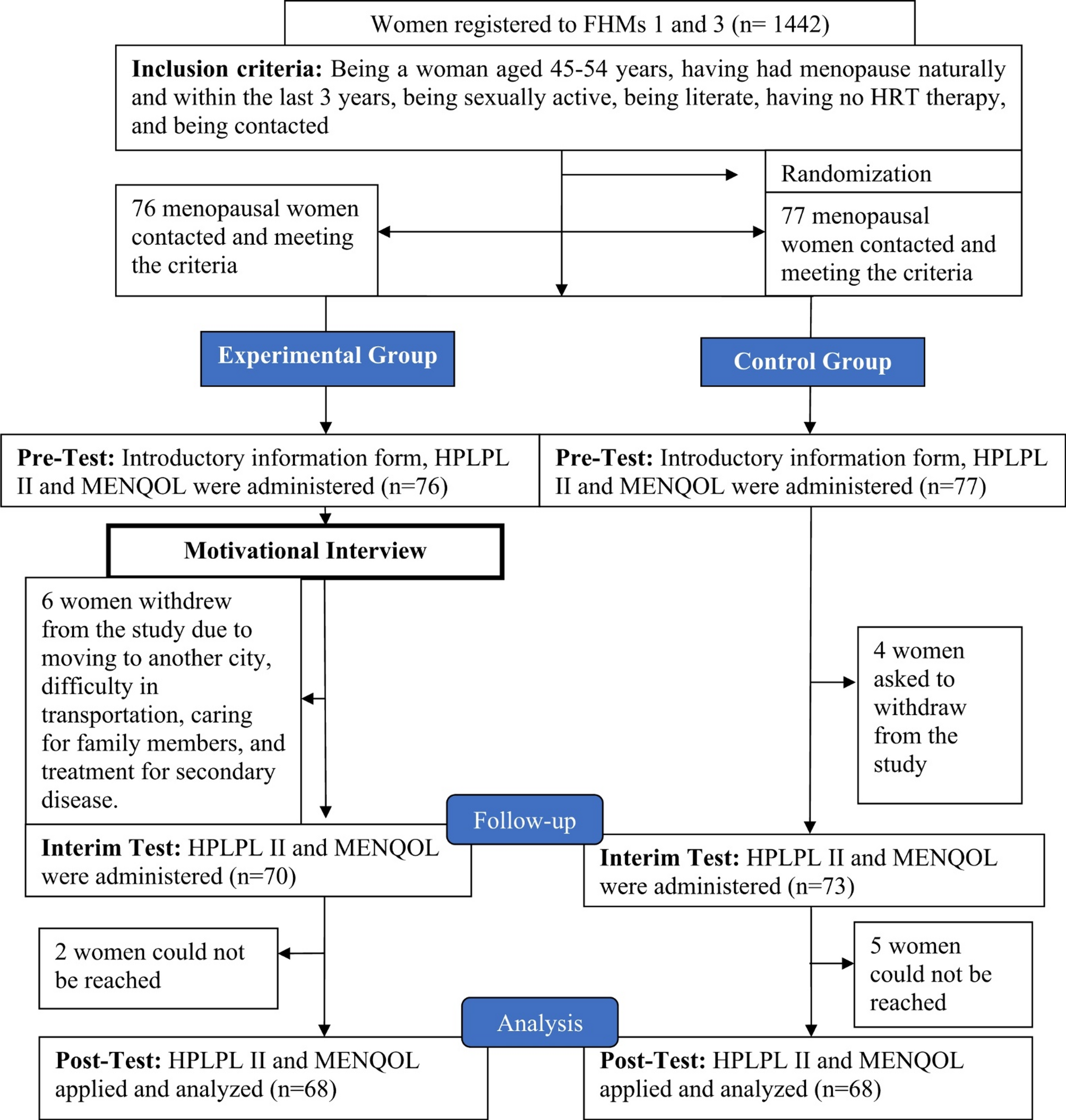
Study design
This randomized controlled trial was conducted in two different Family Health Centers (FHCs) in a province in eastern Turkey. A schematic of the experimental design is given in Fig. 1. This randomized controlled experimental design was conducted in accordance with the CONSORT checklist steps.
Setting and sample
The sample size for this study was determined by G power analysis. The population of the study consisted of 1442 women aged between 45 and 54 years and registered to Family Health Centers No. 1 and 3 in a city center, according to the updated data from Tunceli Provincial Health Directorate in 2018. The sample of the study was determined based on a similar study [11], using the Menopause-Specific Quality of Life (MENQOL) and the Health Promoting Lifestyle Profile II (HPLPL II). A power analysis was conducted, using G*Power 3.1.9.2 and considering the total MENQOL score after education, with an effect size of 0.48, standard deviation of 7.22, power of 0.80, β of 0.05, and α of 0.05. Accordingly, the sample was determined to consist of 136 menopausal women, including 68 in the experimental group and 68 in the control group. A computer-generated random number table was used, which can be used when the sample size is n < 100. For selecting women in the sample, those in the age group of 45–54 years were numbered according to their registration number in the FHCs, using simple random sampling method and a random number table. To prevent cross-contamination between the groups, data for the experimental group were collected from the Health Center No. 1, and data for the control group were collected from the Health Center No. 3. Similarly, in FHC No. 1 and 3, there are 4 physicians, 4 family health personnel, 2 auxiliary health personnel, 4 polyclinics, 1 nurse room, 1 vaccination room, 1 pregnant-baby monitoring room, 1 intervention room and 1 training room. During the practice, no intervention regarding MG was performed on menopausal women in FHCs.
Ethical approval
For conducting the study, an ethical approval was obtained from the Munzur University Scientific Research and Publication Ethics Committee (Date: 03/09/2018/4579) and written permissions from the Tunceli Provincial Health Directorate and the responsible physicians of Health Centers No. 1 and 3. The participants were informed about the purpose of this study, and their written informed consent was obtained using an informed consent form. Compliance with ethical principles was ensured at every stage of the study. Additionally, permission was obtained for using both HPLPL II and MENQOL. No interventions were conducted on women in the control group until the experimental research was completed. After the post-test was administered to women in the experimental and control groups, those in the control group also participated in a motivational interviewing session upon their wishes.
Participants
Between August 15, 2018, and December 30, 2019, the eligibility of women registered with two different FHCs in the city center of Tunceli was evaluated for inclusion in this study. A total of 8 women from the experimental group, 6 women due to transportation difficulties, moving to another city, caring for family members, treatment of secondary disease, and 2 women due to inaccessibility, were excluded from the study. In addition, a total of 9 women from the control group were excluded from the study 5 of them could not be reached and 4 did not participate in the post-test without submitting any reason.
Inclusion criteria
The inclusion criteria were as follows: (1) being literate; (2) being able to make a conscious decision to participate in the study, being able to communicate verbally, and being able to sign a consent form; (3) having had menopause naturally and within the last 3 years; (4) being sexually active; (5) having no hormone replacement therapy.
Exclusion criteria
The exclusion criteria were as follows: (1) unwilling to continue the study; (2) having a psychiatric diagnosis according to the FHC records.
Termination criteria of the study
The termination criteria were as follows: (1) intending to leave the study and (2) move to another city.
Study protocol
The search was randomized by a computer programming module (http://www.randomizer.org/form.htm) designed for controlled trials; a randomization list was prepared, and the participants were randomly assigned either to the experimental group (n = 68) or to the control group (n = 68).
Instruments for evaluation
The following measurement tools were used in this study.
Introductory information form
The introductory information form was prepared by the researcher in line with the literature [24,25,26,27,28,29,30]. It consists of a total of 10 questions about the menopausal women’s socio-demographic characteristics (age, education level, income level, place of residence for the longest time) and history of menopause (duration of being in menopause, training on menopause, thoughts about menopause and coping methods for menopausal complaints).
Health promoting lifestyle profile II (HPLPL II)
The HPLPL II was prepared by Walker et al. in 1987 [31]. This scale is suitable for use in research to evaluate health promotion behaviors within the framework of the health promotion model [11]. The scale includes six dimensions, namely, nutrition, physical activity, stress management, interpersonal relationships, responsibility for health, and spiritual growth (52 items in total). The items are scored based on a 4point Likert scale (never, sometimes, often, and usually). The total score of the scale ranges from 52 to 208. The score of each dimension is calculated separately and a higher scores mean better health. The Cronbach’s alpha coefficient was found as 0.92 for the total scale and ranged between 0.64 and 0.80 for its dimensions, suggesting that the Turkish version of the scale has sufficient validity and reliability [32]. In the present study, the Cronbach’s alpha value of the scale was 0.88.
Menopause-Specific quality of life (MENQOL)
The MENQOL was developed by John R. Hilditch et al. in 1996 to create a health-specific quality of life scale for menopausal women [33], and its Turkish validity and reliability study was conducted by Kharbouch and Şahin in 2007 [34]. This is a 7-point Likert-type scale containing 29 items and consists of four domains: vasomotor, psychosocial, physical and sexual. Each item is scored from “0” to “6”, where “0” refers to “not bothersome” and “6” to “extremely bothersome”. A higher scale score indicates greater severity of the complaint. The Cronbach’s alpha coefficient of the scale domains was found to range between 0.81 and 0.89 [34]. In the present study, the Cronbach’s alpha value of the scale domains ranged between 0.71 and 0.83.
Nursing interventions
Since the method is a technique that generally requires expertise, it is recommended that practitioners undergo a certain training and certification process in order to apply the technique effectively. The researcher participated in the motivational interviewing program and received a certificate prior to the application in this study. Additionally, expert opinions were received from 5 experts during the preparation of motivational interviewing steps. The nursing intervention was applied to 68 menopausal women included in the experimental group. A total of 9 sessions were conducted, including 1 preparation session, 6 motivational interviews, 1 initial follow-up interview one week after the intervention, and 2 follow-up interviews 4 weeks after the initial follow-up. Considering the interactive training method, the motivational interviewing sessions were conducted in the training room of the Health Center in groups of 10 at three different days (Monday, Thursday, Friday) once a week, through face-to-face sessions each lasting 50–60 min. During the interviews, the women were provided with counseling to activate their own sources of motivation, develop healthy lifestyle behaviors and improve their quality of life specific to menopause. The specific contents of the interventions were as follows:
During the research period, no interventions were applied to women in the control group by the researcher, and they filled in the data collection forms simultaneously with those in the experimental group. During the application, no interventions of motivational interviewing were conducted for menopausal women at the FHCs.
Statistical analysis
The data were coded and statistically analyzed using the Statistical Package for Social Science (SPSS 24) software package. Fisher’s exact test and chi-square test were employed by the researcher to determine the homogeneity of women in the experimental and control groups. The effects of healthy lifestyle behaviors and menopausal-specific quality of life between the groups were analyzed using independent samples t-test and repeated measures ANOVA. Cohen’s d value was calculated to determine the effect size for women in the experimental and control groups. The results were evaluated at a 95% confidence interval and a significance level of 0.05.