Attitudes of the Lithuanian public and experts towards the return of IGF
The results of the quantitative study revealed a high level of interest of the Lithuanian population in obtaining information relevant to their health from the biobank. Our survey results align with the attitude surveys of the public and biobank participants from some other countries [14], the overall rate of those interested is substantially high.
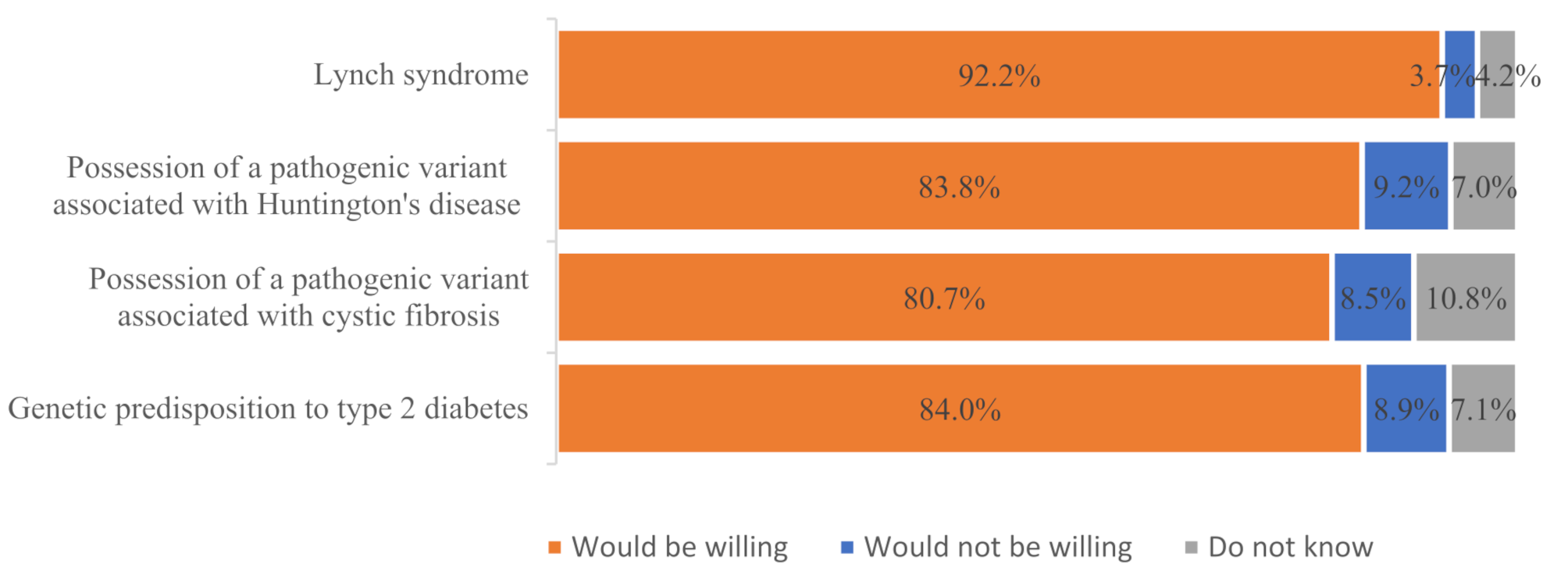
The findings from our study as well as from some other countries reveal that people desire various health-related insights, including information on the risk of untreatable monogenic diseases like Huntington’s disease (PAG approach), the risk of monogenic diseases in offspring (such as possessing a pathogenic variant associated with cystic fibrosis) (PAG approach), and even slight genetic risks for multifactorial diseases like type 2 diabetes (DTC GT approach) [1, 3]. For instance, in the U.S. study, 95% of the 4,659 respondents agreed that they would like to know about health risks related to treatable diseases (e.g., asthma), and 90% expressed interest in learning about risks related to untreatable diseases (e.g., Alzheimer’s disease) [3]. Similarly, in a Japanese study, more than 80% of the population biobank participants expressed a desire to receive information encouraging lifestyle changes, a number even higher than those who wished to receive clinically significant findings (over 50%) [1].
Despite the expressed willingness of the general population to know the information related to their individual health, which was revealed during the biobanking activities, it is important to underline that the results of the qualitative research carried out by the authors revealed a less than uniform attitude of the experts regarding the appropriateness of returning these findings. While experts unanimously agreed that biobank participants should be informed about findings indicating an increased risk of a treatable monogenic disease like Lynch syndrome (MAG approach), they held varying opinions regarding the disclosure of other findings, such as those related to Huntington’s disease (PAG approach), possession of a pathogenic variant associated with cystic fibrosis (PAG approach), and type 2 diabetes (DTC GT approach). The experts, regardless of their professional field, presented arguments both for and against returning findings like possession of pathogenic variant associated with Huntington’s disease or cystic fibrosis to the biobank participants. They highlighted the challenges in establishing a clear policy for informing biobank participants about these findings and changed their views on the appropriateness of returning them accordingly during the interviews. This undoubtedly reflects the complexity of the issue and the need for a debate on the return of IGF. A slightly clearer division of opinion between the domains was observed with regard to the return of the type 2 diabetes finding. When discussing this finding, the most significant differences of opinion were found between the experts according to their area of expertise. Medical geneticists, natural scientists and ethicists considered it inappropriate to return such a finding to a biobank participant. This reflects more a concern that findings that may not be sufficiently informative may cause misunderstanding and confusion for the biobank participant. Conversely, legal and data protection experts considered the return of such a finding to the biobank participant to be appropriate. The latter experts’ position is more reflective of the public’s view.
Similar findings regarding the divergence between public and expert opinions on other findings than the MAG approach suggests, have been observed in contexts unrelated to biobanking. For example, a study on the Danish population in the context of clinical genome sequencing reveals that the general public’s preferences for reporting differ significantly from those of professionals, as indicated in the ACMG guidelines. The general population places greater importance on findings from the PAG approach, which includes severe but clinically non-actionable findings, compared to the MAG approach favored by professionals. This may suggest the need for a new policy that combines elements of both the MAG and PAG approaches to better align with public preferences while maintaining professional standards [15].
The general public and the experts in Lithuania agreed that the finding indicating an increased risk of developing a treatable monogenic disease (Lynch syndrome), discovered during the biobanking activity, should be offered/returned to the biobank participant. However, it is important to note that the Lynch syndrome case scenario within our research study involved a pathogenic variant with relatively high penetrance, which is not always the case and may depend significantly on family history [16]. Therefore, it would still be valuable to explore how both experts and the public perceive low-penetrance monogenic variants, as this is crucial for understanding how opportunistic screening can lead to false positives, overdiagnosis, unnecessary surveillance, and distress.
Lithuanian experts also emphasized the need to validate findings in an accredited laboratory and assess their clinical validity, even though this criterion was not explicitly mentioned in the interview guide. The reason for raising this point is that it poses a significant challenge to both the findings and their practical application, given the substantial resource demands involved in validating all potentially relevant variants in a biobank before they can be returned.
Both issues—the agreement on the disclosure of high-penetrance, serious monogenic diseases and the importance of clinical validity—are already, to some extent, reflected in the current Lithuanian strategy for the return of health-related findings to a biobank participant. Nevertheless, the analysis of the empirical data leads to a number of points to be addressed and improved. Firstly, as the results have shown the disagreements may arise between experts on the assessment of specific findings against the established strategy. However, it may be even more difficult for biobank participants to understand what health information they can obtain from a biobank. In other words, once a biobank participant has been informed of a finding, he or she may still be surprised that he or she has agreed to be aware of the finding. Secondly, Lithuanian experts and citizens considered other criteria not set out in Lithuanian legislation (e.g. invasiveness of the preventive measure) to be important in the decision to know/return a particular finding. Thirdly, experts and the public highlighted that while all the criteria discussed (severity of disease, likelihood of disease, effectiveness of the preventive measure and invasiveness) may be relevant when considering whether to return/know a particular finding, each of these criteria may have a different weight in this decision. It is therefore important to consider the interplay between all criteria when deciding whether to know/offer a particular finding.
Measures to improve the IGF strategy in Lithuania
Based on the results of empirical research, as well as the discussion of these findings, the authors of this paper believe that specifying the current strategy for returning IGF to biobank participants in Lithuania—while still following the MAG approach—should be considered the primary goal for improving the return of IGF from biobanks. This could be done in at least two ways.
Development and use of a list of genes and diseases
The genes and diseases included in this list should be selected based on criteria deemed important by Lithuanian experts and the public. The advantages and challenges of using such a list in biobank activities are presented in Table 8.
The list of genes and diseases is recommended and/or applied in several scientific clinical projects in other European countries that aim to integrate genome sequencing into clinical practice [17, 18]. In recent years, this method has also been adopted in biobanks [19, 20]. One of the advantages of using a gene and disease list is that it makes it easier for all individuals involved in biobank activities (e.g., biobank administrators, participants, and funders) to understand which findings might be detected and returned to biobank participants. For participants, reviewing the gene and disease list can be helpful in reducing unrealistic expectations about the information they might receive, such as avoiding the misconception that not finding any results means they are in good health. For biobank administrators and researchers, this list is a convenient tool for limiting the number of findings that may be returned during biobank operations. Moreover, it simplifies the process for those managing and funding the biobank to calculate and plan the necessary human and financial resources to effectively implement the return of findings [21]. It is also worth noting that the adaptation and application of a gene and disease list require minimal changes to the legal regulations in Lithuania.
Despite the advantages of applying a gene and disease list, there are also challenges associated with its implementation. First, creating and regularly updating such a list requires expert knowledge, time, and financial resources, which are very limited for conducting biobank activities in Lithuania. One possible solution to this challenge is to use existing gene and disease lists. For example, this approach has been adopted by the Estonian Biobank, which uses the gene and disease list prepared and continuously updated by the American College of Medical Genetics and Genomics (ACMG) [22,23,24,25,26] as one of the methods for evaluating findings. However, it is important to note that while the ACMG gene and disease list is becoming a standard for the return of findings in various scientific projects, the specific needs of a biobank—considering factors such as the focus of planned research, the characteristics of the biobank’s participant population, the intensity of communication with participants, and the resources available for implementing a return of findings strategy—might make a narrower or broader list of genes and diseases more appropriate [21].
A further challenge in applying a gene and disease list lies in the limited capacity of researchers to curate and interpret findings. Gene lists may indicate which results warrant consideration for return, but they do not determine which policy should be implemented. For example, policies may require researchers to actively screen all listed genes for pathogenic variants—an approach that may prove unfeasible in many research contexts—or to report such findings only if they are incidentally discovered in the course of research, which could be a more practical approach.
One more complication in applying a gene and disease list concerns the assumptions regarding clinical responsibilities. In the article we referred to duties such as the duty to rescue or obligations that apply when the researcher is also a medical doctor. However, it is important to clarify that many genomics researchers are not clinicians and have no direct relationship with participants. As a result, they may not bear the same ethical or professional responsibilities as healthcare providers. This may complicate the application of clinical norms in research settings. One way to address this gap is by integrating clinical team members into the research team from the outset, thereby ensuring that appropriate expertise is available and responsibilities are clearly defined where needed.
Another challenge related to the use of a gene and disease list is that researchers might discover other findings (not included in the list) that they consider significant for the participant’s health. This challenge could be mitigated by establishing an advisory body for the biobank, which would be responsible for reviewing new cases of findings not included in the list.
Use of guidelines for evaluating criteria for returning IGF
These guidelines can serve as an alternative to the previously discussed gene and disease list or as a supplementary tool to help determine which genes and diseases should be included in the list. One example of such guidelines is the five-criteria scale proposed by Berg and colleagues for assessing the clinical significance of specific genetic conditions. This scale was developed by an interdisciplinary group of experts, including not only clinical geneticists but also specialists from other fields (e.g., cardiology, neurology, primary care), clinical laboratory professionals, and ethics experts. The criteria in this scale include the severity of disease outcomes, the probability of disease occurrence, the effectiveness of interventions, the burden of interventions, and the level of evidence, with scores for these criteria ranging from 0 to 15 in total. A higher total score across these five criteria indicates greater clinical significance of the genetic variant [27].
The scale proposed by Berg and colleagues for determining the clinical significance of findings might be appealing to those involved in biobank activities (Table 9). This is primarily because the scale provides a rationale for why a particular finding is or isn’t returned to a biobank participant. Using this tool could also contribute to a more transparent and consistent evaluation of findings. Additionally, it is easily adaptable to different return-of-findings strategies and contexts. For example, while Berg and colleagues suggest focusing solely on genes associated with monogenic health disorders, the tool can be readily adapted to evaluate genes associated with complex diseases if needed. It is also worth noting that, like the gene and disease list, integrating this scale into the return-of-findings strategy in Lithuania would require only minimal legal adjustments.
One of the major challenges with using this scale is that the interpretation of its criteria and the assignment of scores might vary among experts from different fields. Therefore, it would be advisable to establish an interdisciplinary advisory body for the biobank to assist in evaluating specific findings using this scale.
Given the significant variation in Lithuanian experts’ opinions regarding the return of non-clinically actionable health information and the high level of willingness expressed by the general population, the residents to receive such information, it is important to continue developing discussions and conducting empirical research on this topic. For instance, understanding why the Lithuanian public wants non-clinically actionable health information and the psychological aspects of returning such information could be valuable. This issue, along with the broader question of IGF returning, could be examined not only within the context of biobanks but also in the broader context of healthcare.
Study limitations
We recognize that the empirical study outlined above has certain limitations. One significant limitation of the qualitative study is that many scientists involved in the research, who work with samples and health data stored in the biobank, also hold additional roles related to the biobank, such as founders or managers. While the perspectives of these experts are particularly valuable, their views on the investigated aspects may differ from those of scientists who do not have an inter-dependent relationship with the biobank. Therefore, it would be essential to explore the viewpoints of this other group of researchers in future studies.
The quantitative study also presents several limitations. Firstly, the survey was conducted among members of the Lithuanian population who voluntarily agreed to participate, resulting in a relatively low response rate of 22.7%. While this outcome was anticipated, we aimed to enroll as large as possible sample from the invited ones. However, it remains uncertain if the views of those who chose not to participate might have affected the study results.
Secondly, the study focused on hypothetical scenarios rather than actual human behavior. It is important to note that individual behavior may vary based on contextual factors, such as personal experiences with the healthcare facility associated with the biobank or the specific circumstances under which they were invited to participate.
Thirdly, since this study involved the general population in Lithuania than biobank participants and most surveyed individuals reported their health as fair or good, the findings may be more applicable to population-based biobanks rather than disease focused ones.
Finally, while the sample was designed to be representative concerning gender, age, place of residence, and education, there were challenges in ensuring the participation of older adults (65+) and those with lower education levels (e.g., primary education). These groups tend to be less technologically literate and less likely to use computers, resulting in their underrepresentation in the survey, and data weighting did not help to overcome this problem.