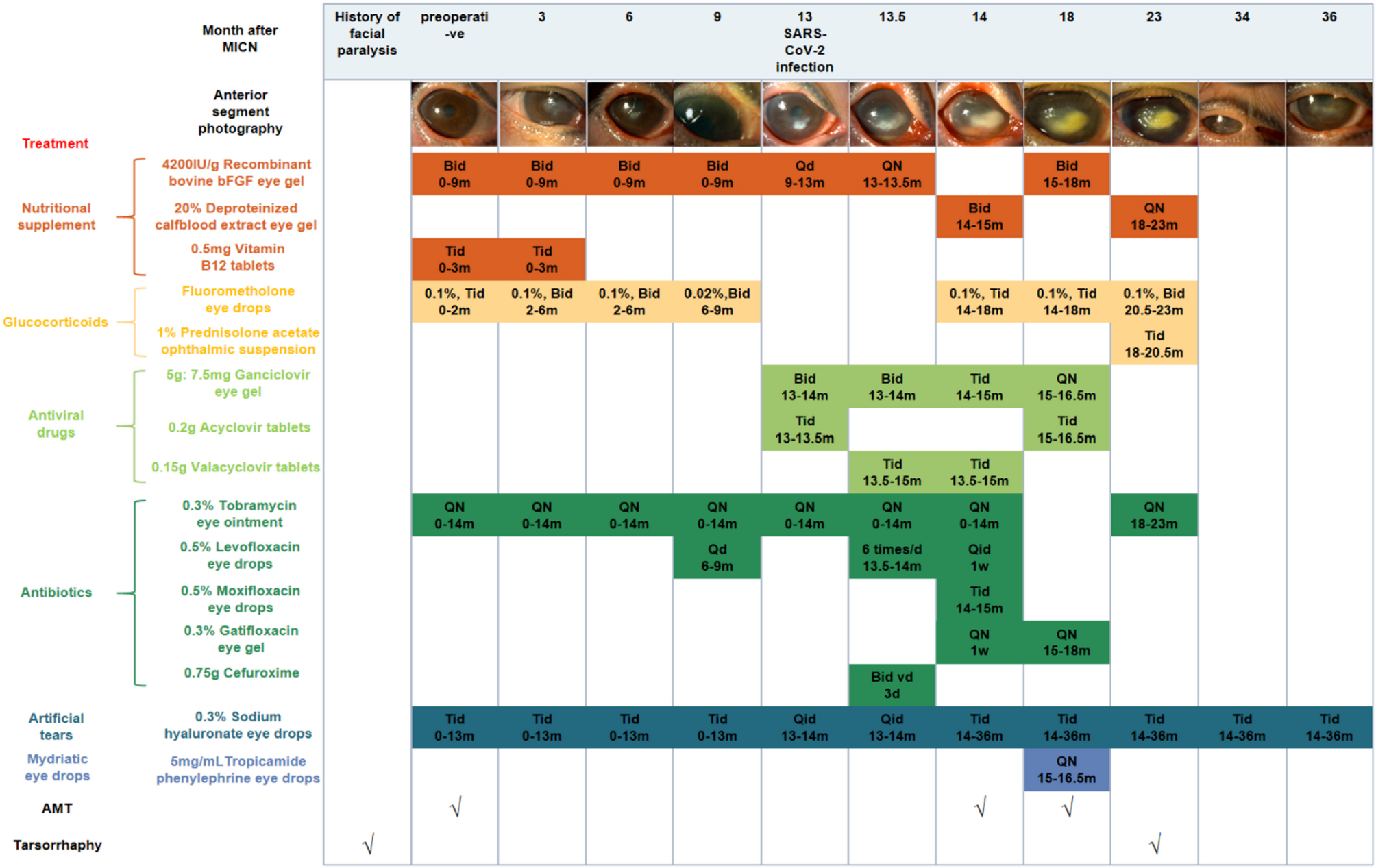
Initial visit
In December 2021, a 59-year-old woman presented to Shanghai Ninth People’s Hospital with a 1.5-year history of visual acuity decline in her right eye. She had a prior history of resection of a right-sided acoustic neuroma at an external medical facility, complicated by right facial paralysis (House-Brackmann Grade VI) and NK (Mackie stage III). She had undergone right eyelid tarsorrhaphy fifteen months earlier and facial nerve transplantation with nerve anastomosis four months prior. She denied any history of diabetes mellitus, AIDS, or autoimmune conditions such as rheumatoid arthritis.
On examination, the right eye demonstrated a central corneal lesion characterized by epithelial erosion, stromal edema and thickening, and neovascularization. The best-corrected visual acuity (BCVA) was 0.01, and corneal sensation was 0/60 using the Cochet-Bonnet esthesiometer (Luneau Ophtalmologie, Chartres, France). The Cochet-Bonnet esthesiometer stimulates mechanoreceptors and polymodal nociceptors, which constitute approximately 90% of corneal nociceptors [5]. Corneal sensation testing was conducted by the same examiner. During the assessment, the patient was seated indoors under natural lighting, initially fixating straight ahead. The examiner applied perpendicular pressure to the central cornea, starting with a filament length of 60 mm and decreasing in 5 mm increments. The maximum filament length eliciting a positive response was recorded as the corneal sensitivity threshold, with three consecutive verifications [6]. Subsequently, she was instructed to sequentially gaze into each of the four peripheral quadrants to evaluate corneal sensation in each region. Results were documented in millimeters of filament length.
In vivo confocal microscopy (IVCM) examination was conducted utilizing the Rostock Cornea Module of the Heidelberg Retina Tomograph II (HRT II RCM, Heidelberg Engineering GmbH, Heidelberg, Germany). IVCM demonstrated a significant reduction in subepithelial nerve fibers, with most regions lacking nerve innervation. She was diagnosed with Mackie stage III NK. Given the refractory nature of her NK and inadequate response to conventional therapy, she was indicated for corneal neurotization.
Minimally invasive corneal neurotization
Corneal neurotization procedures are primarily categorized into direct corneal neurotization (DCN) and indirect corneal neurotization, known as MICN [7, 8]. In DCN, the supratrochlear and supraorbital nerves are identified via a coronal incision at the vertex and subsequently dissected [9, 10]. The principle of MICN involves anastomosing the contralateral supraorbital nerve and supratrochlear nerve with a free sural nerve graft through a minimally invasive approach [11, 12]. Approximately 12–14 cm of sural nerve is harvested as the donor nerve [11, 12]. A 2-cm incision is made in the upper eyelid to isolate the nerve branches of the trochlear and supraorbital nerves [12, 13]. The choice of surgical technique depends on the patient’s condition, the extent of trigeminal nerve damage and the availability of donor nerves. The patient had previously undergone craniotomy for acoustic neuroma resection, and DCN could potentially increase the risk of complications such as encephalitis [10]. In DCN, dissection of the supraorbital nerve may result in forehead hypoesthesia. A section of sural nerve was easy to obtain as the donor nerve in this case. Our hospital was the first medical center in China to perform MICN, with the largest case volume to date. Given that both approaches are accepted treatments for NK with comparable clinical outcomes at twelve months, MICN was considered to cause less trigeminal nerve dysfunction and was deemed safer and technically feasible for this patient without compromising therapeutic goals [10]. Thus, she timely underwent MICN and amniotic membrane transplantation (AMT).
Serial examinations at one to three months post-MICN demonstrated gradual but significant improvement in corneal epithelial integrity and stromal clarity (Fig. 1). Corneal sensation in the temporal quadrant improved to 5/60 (Table 1). IVCM revealed elongated, continuous nerve fibers with improved longitudinal trajectories, suggesting nerve regeneration (Table 2). Corneal nerve fiber length (CNFL) refers to the total length of corneal nerves per mm2.Five representative images of the central corneal sub-basal nerve plexus and dendritic cells (DCs) were selected for quantitative analysis based on criteria including the complete image of the same layer, optimal contrast and maximal visibility of nerve fibers and DCs. Sub-basal nerve plexus analysis was performed using an automated software ACCMetrics (MA Dabbah, Imaging Science and Biomedical Engineering). DC density was calculated as the total number of DCs per image and was analyzed using ImageJ. Postoperative corneal sensation and nerve density gradually increased. During the first three postoperative quarters (nine months) following MICN, her corneal epithelium, sensation and nerves gradually recovered (Tables 1 and 2).
Pictures of anterior segment photography and treatment of the patient. Abbreviations: bFGF, basic fibroblast growth factor
Postoperative care included: nutritional supplementation (corneal nerve regeneration), glucocorticoids (inflammation control/immune rejection prophylaxis), nocturnal antibiotic ointment (exposure/infection prevention), and artificial tears (adjunctive therapy) (Fig. 1). Low-concentration glucocorticoids were withdrawn at nine months post-surgery.
SARS-CoV-2 infection episode
Thirteen months post-MICN, the patient developed COVID-19 confirmed by antigen testing, manifesting as a self-limited upper respiratory tract infection. Within one week of viral onset, she experienced acute visual deterioration, representing disease recurrence. Ocular examination revealed a white infiltrative corneal lesion adjacent to the limbus at the 4–5 o’clock position in the right eye, characterized by well-demarcated borders, epithelial defect, stromal edema, and increased purulent secretion (Fig. 2). IVCM revealed inflammatory cell infiltration in the lesion area, and no fungal hyphae or other pathogens were detected. Based on clinical experience, we initially suspected that the patient had infectious keratitis, possibly caused by a viral or bacterial infection. Eye drops for antibacterial and antiviral treatment were administered to the patient. However, two weeks later, the corneal ulcer progressed and there was hypopyon. IVCM findings indicated sparse subepithelial nerve fibers, with most regions lacking nerve presence. Additionally, DC density increased from 41.80 ± 26.13 n/frame to 88.60 ± 20.55 n/frame within two weeks (Table 2).
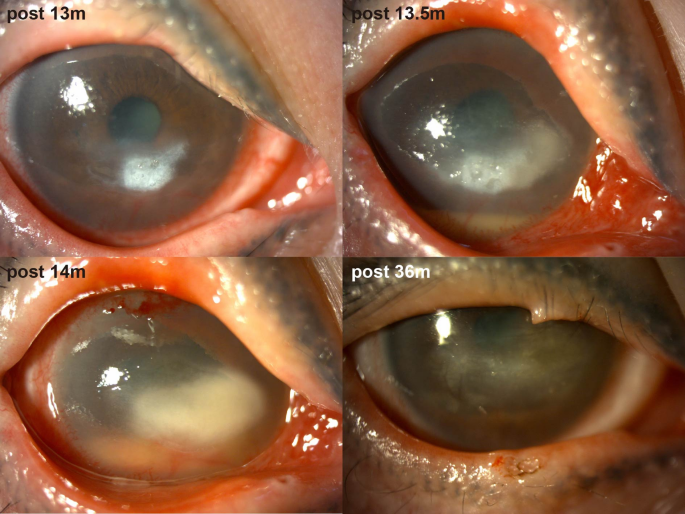
Pictures of anterior segment photography of the patient postoperatively after SARS-CoV-2 infection
Given the progressive corneal ulceration and hypopyon, she underwent ocular debridement of the right eye and anterior chamber paracentesis for microbiological diagnostics. The anterior chamber was irrigated, and aqueous humor samples were collected for high-throughput sequencing. Corneal exudate culture results were negative and no pathogenic microorganisms were identified through sequencing analysis. Comprehensive autoimmune serology showed normal levels of autoimmune markers and antibodies. Based on the characteristic clinical manifestations, response to treatment, and temporal association with confirmed SARS-CoV-2 infection, a presumptive diagnosis of COVID-19-related NK was established, representing a virus-related keratopathy.
Protracted neurotrophic keratopathy following SARS-CoV-2 infection
Management of COVID-19-related NK comprised: (1) antiviral agents (e.g., ganciclovir) to mitigate herpesvirus reactivation risk potentiated by SARS-CoV-2-induced immune dysregulation; (2) nocturnal antibiotic ointment/gel for corneal exposure protection and infection prophylaxis; (3) nutritional supplementation; (4) glucocorticoids for inflammation control; (5) artificial tears; (6) 5 mg/mL tropicamide phenylephrine eye drops for anterior chamber inflammation prevention; and (7) two AMTs (Fig. 1). During a severe inflammatory phase (approximately 2 months), 1% prednisolone acetate ophthalmic suspension superseded 0.1% fluorometholone therapy. Four months later, the hypopyon subsided, and the lesion became localized but remained Mackie stage III (Fig. 1). Five months later, central tarsorrhaphy was performed. She did not come for follow-up for a year due to personal excuses. Twenty months after SARS-CoV-2 infection, she showed up and reported alleviation of ocular symptoms. Examination revealed the transparent cornea with only residual corneal macula (Fig. 2). Corneal sensation increased to 30–40/60. During the subsequent follow-up, the patient’s condition remained stable and the NK healed, so lysis of tarsorrhaphy was performed.
Reviewing the treatment process, the patient’s corneal sensation exhibited a trajectory characterized by complete loss, partial recovery, relapse, and eventual partial recovery. Corneal nerve innervation shifted from denervation to neurotization, reverted to denervation following viral infection, and ultimately returned to neurotization (Fig. 3 A, B). DC density initially increased, then decreased, and subsequently rose again post-viral infection (Fig. 3 C, D). DCs played dual roles: facilitating corneal nerve regeneration after MICN and aggravating nerve injury via immune-mediated mechanisms following SARS-CoV-2 infection [4, 14]. Following resolution of recurrent NK after MICN, corneal nerve regeneration was observed, demonstrating that MICN effectively promoted corneal nerve regeneration. Neural pathways reconstructed through peripheral nerve transplantation can regenerate despite prolonged viral infection.
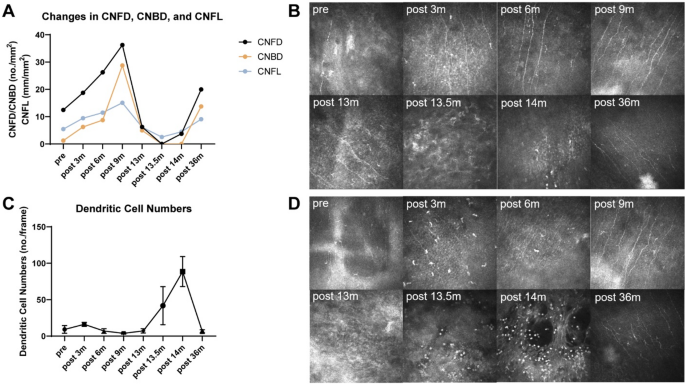
In vivo confocal microscopy outcomes of the patient. A Changes in CNFD, CNBD, and CNFL of the patient. B In vivo confocal microscopy images of corneal nerves. At 36 months postoperatively, the area with the highest density of corneal nerves was not captured during in vivo confocal microscopy examination due to the patient’s right-sided tarsorrhaphy. C Changes in corneal dendritic cell numbers of the patient. (D) In vivo confocal microscopy images of corneal dendritic cells. Abbreviations: CNFD, corneal nerve fiber trunk density, the number of fibers per mm2 (no./mm2); CNBD, corneal nerve branch density, the number of branch points on the main fibers per mm2 (no./mm2)