A 34-year-old woman of childbearing age came to the local hospital for her annual well-woman visit, and an ultrasound revealed a cystic solid pelvic mass. The patient was subsequently admitted to our hospital. The patient exhibited no symptoms indicative of abdominal discomfort, vaginal bleeding, change in bowel habits, or change in urination. The absence of clinical manifestations precluded determination of its onset time or progression timeline. Moreover, she had undergone one cesarean section and had no history of uterine fibroids. Furthermore, the patient reported no prior use of hormonal treatments or oral contraceptive pills. Additionally, she had no significant history or family history of hypertension or diabetes mellitus, and no history of smoking or alcohol consumption. Her vital signs were within normal limits. A specialized gynecological examination was conducted, during which the uterus was found to be anteriorly positioned, of normal size and shape, and free of tenderness. Additionally, no palpable mass was identified in the left adnexal region, and no tenderness was observed. On the right side, a mass of approximately 5.0 × 6.0 cm was detected in the adnexal region, exhibiting distinct boundaries, a smooth surface, and no evidence of pressure pain.
The blood routine, blood coagulation, liver and kidney function, electrolytes, and glucose levels were within the normal limits. The tumor marker alpha-fetoprotein (AFP) was elevated at 10.90ng/ml. The remaining tumor markers, carcinoembryonic antigen (CEA), carcinoma antigen (CA) 15 − 3, CA 19 − 9, and CA 12 − 5, were within the normal range.
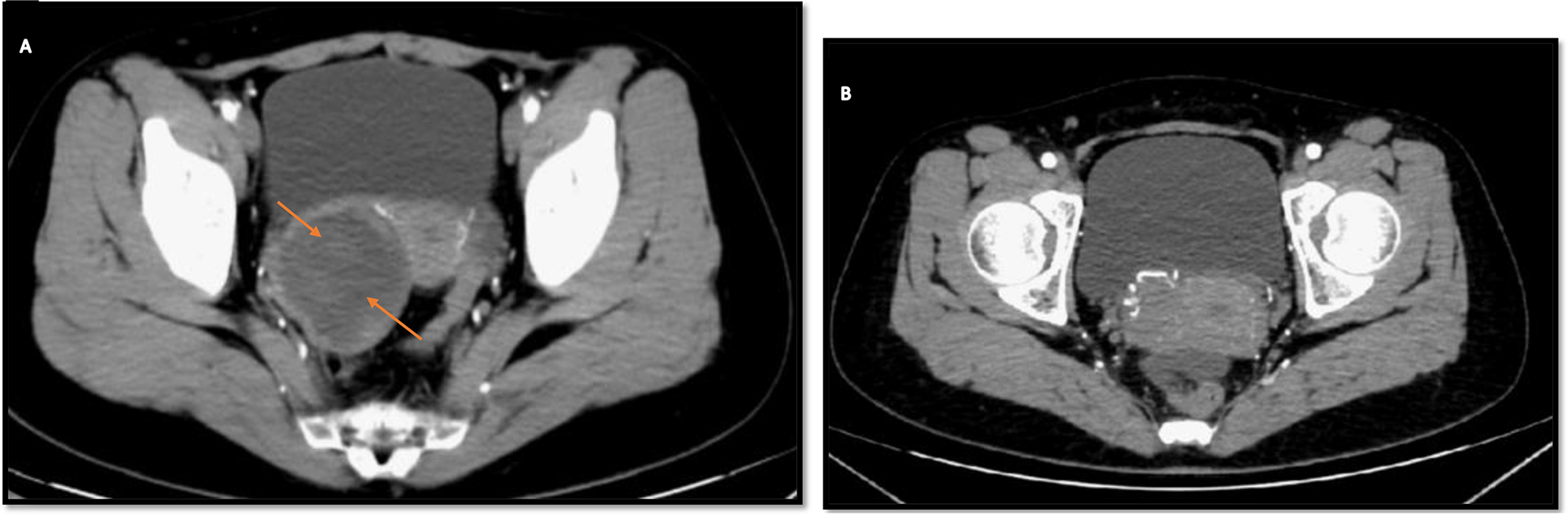
Transvaginal ultrasound demonstrated a cystic solid echogenic mass of approximately 5.2 × 4.5 × 5.7 cm in the right adnexal region with well-defined borders and peripheral blood flow signal in the form of punctate streaks. The bilateral ovaries were visible, and the uterus appeared normal. Given concerns for ovarian malignancy, contrast-enhanced pelvic CT was performed, demonstrating an irregular cystic solid hypodense shadow of about 6.2 × 5.3 cm in the right adnexal area, with a slightly unclear border. Figure 1(A) displays the arterial phase, while Fig. 1(B) indicates that the mass has sufficient blood supply. Contrast enhancement revealed mild, uniform enhancement of the cystic wall and inner solid components, without nodular hyperenhancement. The intracystic fluid region was not of homogeneous density. Rectal walls were normal, with no pelvic lymphadenopathy. Chest CT prior to surgery was unremarkable.
Computed tomography (CT): Shows a cystic solid hypodense shadow of about 6.2 × 5.3 cm in diameter with a slightly poorly defined border. (A) CT image after contrast injection; (B) Rich blood supply to the mass
The patient was a 34-year-old female of childbearing age with a well-defined cystic solid pelvic mass of unknown nature, indications for surgery, and the patient’s desire for surgery. There were no contraindications to surgery, adequate preoperative preparation, and laparoscopic exploration was performed. Intraoperatively, a small amount of yellowish fluid was visible in the pelvis. The anterior position, morphology, and size of the uterus were normal, and no masses were seen in the bilateral adnexa. The peritoneum was intact, and a retroperitoneal mass of about 5.0 × 6.0 cm in size with irregular morphology and a smooth surface was observed on the surface of the right retroperitoneal iliac vessels and ureter. Additionally, anomalous vessels were identified (Fig. 2(A)). A thorough examination of the pelvic, abdominal, and bowel walls did not reveal any evidence of abnormal nodules. The tumor is located on the surface of the ureter and iliac vessels, necessitating meticulous separation to avoid damage. Therefore, we opened the peritoneum close to the root of the tumor, and the tumor margins were clear. The specimen was then removed in a specimen bag (Fig. 2(B)). Intraoperative cytopathology suggested that the tumor was a spindle cell tumor, which was a smooth muscle tumor with cystic degeneration. Macroscopically, the tumor was a 5.0 × 6.0 cm cystic-solid mass with swirling structures visible in profile. In addition, a cystic cavity with clear fluid was observed (Fig. 2(C)). Microscopically, the lesion consisted of spindle-shaped smooth muscle cells with bluntly rounded nuclei at both ends, uniformly fine chromatin, and no apparent anisotropy or nuclear division. Some areas showed cystic degeneration and the tumor cells’ fascicular arrangement. This finding confirmed the intraoperative frozen section diagnosis of a spindle cell tumor, a smooth muscle tumor with cystic changes(Fig. 3(A)). Moreover, the excised margins of the mass were free of tumor cells. Immunohistochemical staining showed tumor cells positive for desmin (Fig. 3(B)), smooth muscle actin, estrogen receptor (ER), and progesterone receptor(PR). However, they were negative for the cluster of differentiation (CD) 34 and CD117, findings consistent with a diagnosis of pelvic retroperitoneal leiomyoma. Notably, we also observed the occurrence of cystic degeneration in tumor cells(Fig. 3(C)).
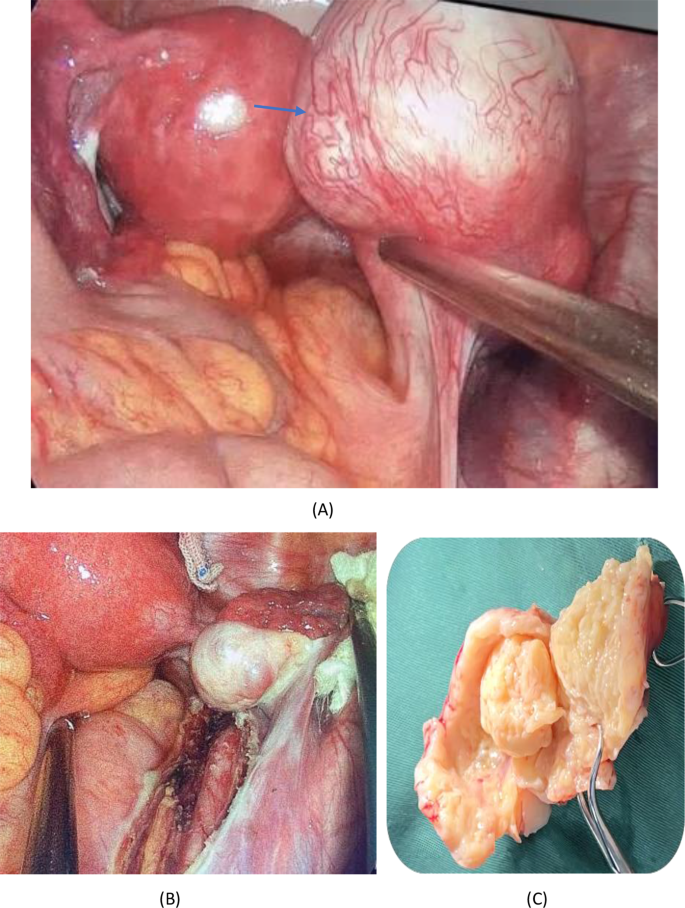
(A) Intraoperative picture showing blood-rich round mass with a smooth surface. (B) The mass traveled on the surface of the right iliac vessels and the right ureter, as seen after complete excision of the mass. (C) The tumor contains a cystic cavity filled with clear fluid, and the cut surface reveals a swirling structure
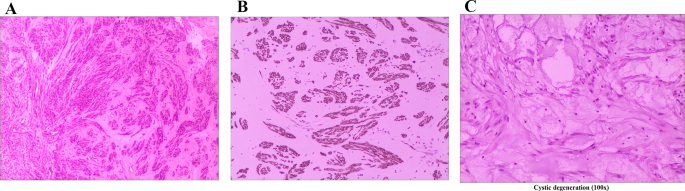
(A) Microscopic examination. Hematoxylin and eosin (HE) staining showed spindle-shaped tumor cells growing in loose bundles against a background of abundant vitreous stroma, cystic degeneration were observed, and no mitoses. (HE,40X). (B) Microscopic examination. The tumor revealed strong positive staining for desmin (100X). (C) Microscopic examination. The tumor showed signs of cystic degeneration (100x)
The patient recovered uneventfully and was discharged on postoperative day 4. Therefore, the patient is required to undergo gynaecological ultrasound examinations at the outpatient clinic on a 3 to 6-month basis post-surgery. No evidence of tumor recurrence was observed on the ultrasound six months after surgery. However, it is important to note that regular follow-up is still necessary.