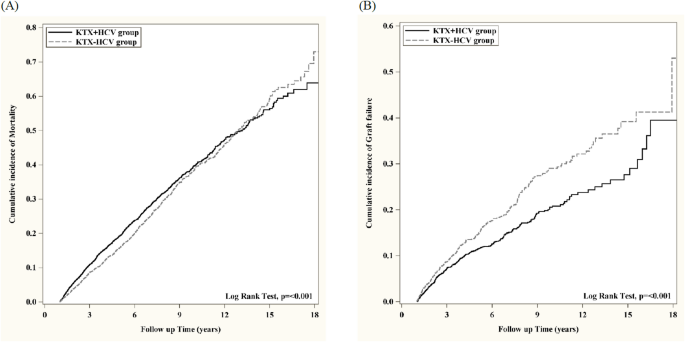
In the present 10-year observational study, associated confounding factors including age, transplant year, sex, and comorbidities, such as hypertension, diabetes mellitus, obesity, primary glomerulonephritis and immunosuppressant agents, were included in a large-scale analysis of a HCV renal recipient cohort that was propensity score-matched with a non-HCV renal cohort. Our findings showed that there was only a slightly higher mortality risk among renal recipients with HCV infection, with a 1.19-fold increased mortality risk. In a previous study, a high mortality and graft failure risk was reported in HCV-infected renal recipients. HCV renal recipients were thus presumed to have a greater likelihood of having an unfavorable outcome after kidney transplantation14. Moreover, a higher risk for death was found during the first 6 months after transplant. However, transplantation still had a superior outcome compared with remaining on dialysis in terms of long-term outcomes in an investigation of patients receiving either transplantation or continuing to receive hemodialysis15. In the present study, we directly compared renal recipients with and without HCV infection, and the findings showed only a slightly increased risk of mortality in HCV infection, suggesting a significant improvement in post-transplantation care for HCV-infected renal recipients. Moreover, our findings showed a favorable survival of renal allograft in HCV infection with a significantly lower risk of graft failure (0.696-fold decrease). This result is consistent before and after propensity score matching, which reached statistical significance. In previous studies, the risk associated with renal allograft loss was remarkably attenuated in multivariable models that included associated confounders, such as longer duration of waiting list, higher PRA, and re-transplantation16. Our findings support results reported in previous studies.
In kidney transplantation with HCV infection, whether HCV infection could increase the risk of acute rejection remains considerably controversial5,14. ,17,18,19,20 In the previous literature, the results were conflicting because certain data, including PRA level, waiting time, and immunosuppressive agents, such as induction therapy, were not assessed, and many studies had small case numbers with a short study period6. In the present study, our results showed a lower risk of renal allograft rejection in HCV-infected recipients. There was a 0.561-fold decreased risk before PSM and 0.694-fold decreased risk after PSM, which both reached statistical significance. These findings are notable as they have never been reported previously. In the case of HCV-infected renal recipients, a previous study suggested that a decreased number of naive T helper lymphocytes, as well as low responses of T helper lymphocytes to stimulation by mitogens would result in an immunodeficient state and that may account for the decreased acute rejection risk in HCV-infected renal recipients6,21. Our results appear to provide solid evidence supporting these findings. Taken together, in comparison to the renal recipients without HCV infection, a distinct improvement of renal allograft survival with a lower graft failure risk and a low rejection risk was noted in this study. The application of modern immunosuppression and superior post-transplant care may account for the better renal allograft results in this study.
Hepatic complications remain a major concern in renal recipients with chronic HCV infection and data on the cause-specific risks of liver cirrhosis, hepatoma, and liver failure post-transplantation remain lacking. Previous studies suggested that HCV-infected recipients had higher death rates caused by liver disease than HCV-negative patients in a univariate analysis22,23,24,25. In the present study, multiple relevant variables were included to eliminate bias to the greatest extent possible through propensity score matching. A comparison of renal recipients groups with and without HCV infection demonstrated that HCV-infected recipients were significantly associated with a remarkably high risk of hepatic complications post-kidney transplantation. A 5.425-fold increased risk was noted before PSM, which was consistent with a 4.128-fold risk after PSM. Moreover, a 12.12-fold increased risk of new onset hepatoma post-kidney transplantation was observed before PSM and there was an 8.957-fold greater risk after PSM. With respect to other hepatic complications, including liver cirrhosis as well as hepatic failure, the trend was consistent and was significantly different. To the best of our knowledge, this is the first study to report the cause-specific risk ratio of post-transplantation hepatic disease, including hepatoma, cirrhosis, and liver failure in renal recipients with HCV infection. To date, the hepatoma risk in HCV-infected recipients has been neglected and data on the hepatoma risk post-transplantation in HCV-infected recipients remain lacking. In one study with a small case number, very few isolated cases of hepatocellular carcinoma (HCC) were reported. It has been proposed that the risk of developing chronic liver disease post-transplantation is closely associated with duration of disease, possibly over 20 years26. A high risk of HCC post-transplantation was observed in the study, which reached a 8.957-fold increased risk in HCV-infected transplantation and this finding has never been reported previously. Our findings indicate that hepatic complications after kidney transplantation remain a major concern in renal recipients with HCV infection, which has to date been neglected.
Our study was focused on hepatic outcomes and did not explore non-hepatic post-transplant complications (e.g., cardiovascular events, new-onset diabetes), which are also clinically important and warrant further investigation in future studies.
Recently, direct-acting antivirals (DAAs) have been demonstrated to dramatically transform the care of patients with chronic hepatitis C virus (HCV) infection3,27,28,29,30. In kidney transplantation, DAAs have been demonstrated to remarkably reduce the risk of death after renal transplantation and that early commencement of DAAs post-transplantation would improve patient and allograft survival among HCV-positive recipients3. Because a high risk of hepatic complication has been demonstrated, early eradication of HCV infection is recommended for patients requiring renal transplantation17.