The question of when people first arrived in the land mass that now comprises much of Australasia has long been a source of scientific debate.
Many Aboriginal people believe they have lived on the land since time immemorial. But until the advent…
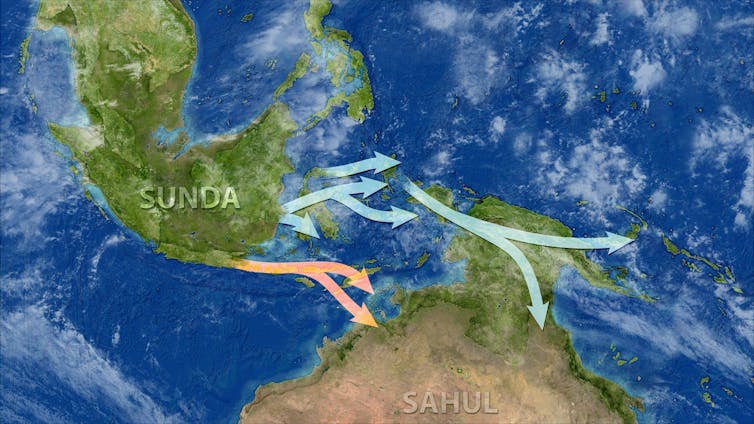
The question of when people first arrived in the land mass that now comprises much of Australasia has long been a source of scientific debate.
Many Aboriginal people believe they have lived on the land since time immemorial. But until the advent…