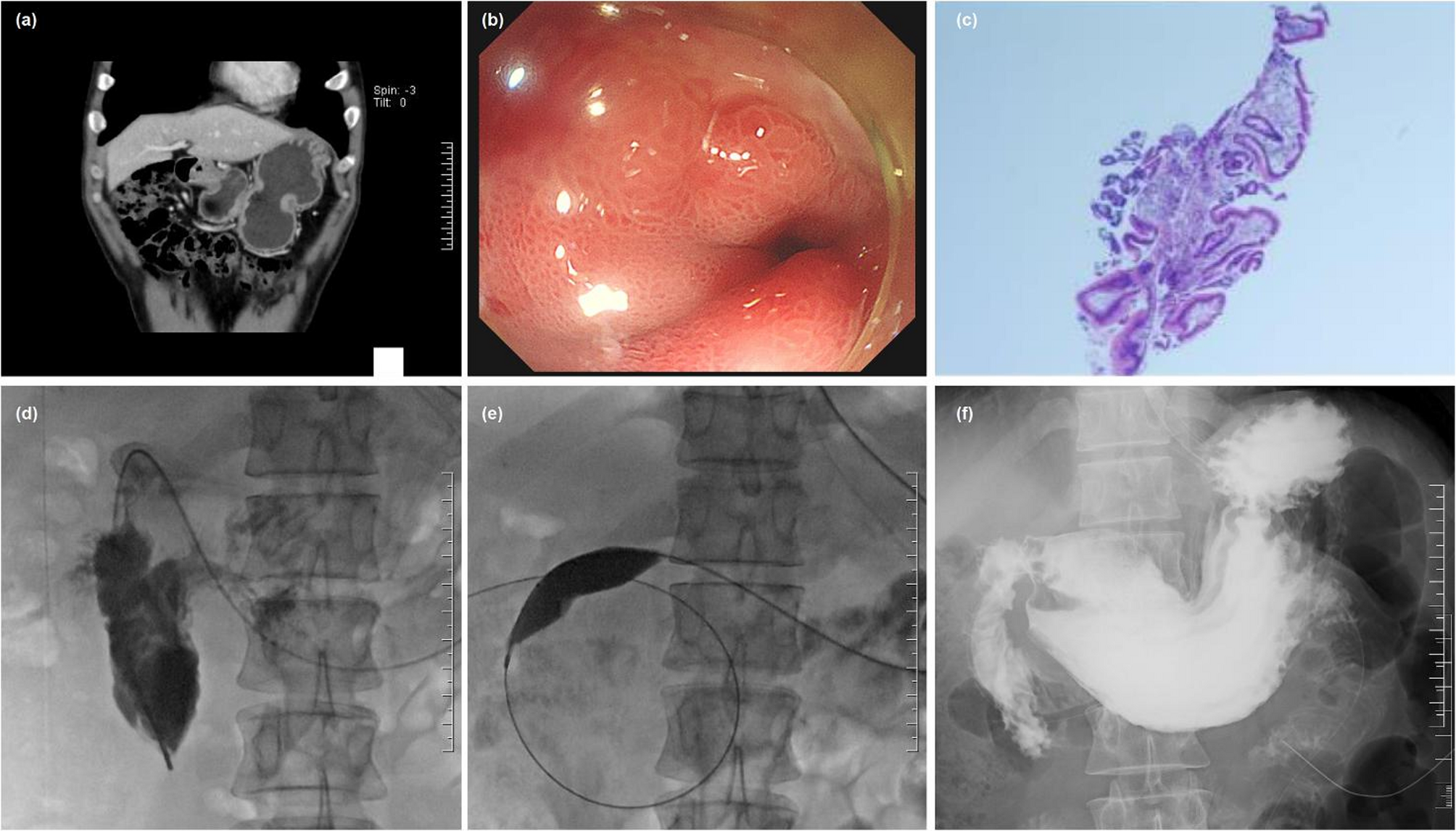
Endoscopic ultrasound-guided gastrojejunostomy (EUS-GJ) is a therapeutic option for GOO [10, 11], which involves directly identifying the jejunum from the stomach and placing a lumen-apposing SEMS between the stomach and jejunum. EUS-GJ can prevent surgical treatment in 83.3% of patients with GOO, and the recurrence rate of GOO after stent removal is as low as 5.6% [11]. However, this challenging technique is usually performed in a few hospital centers, target identification of the jejunum and proper placement of SEMS are challenging steps for this technique. Surgical gastrojejunostomy is a traditional modality for treating gastroduodenal obstruction, which alters the anatomy of gastrointestinal tract, and can serve as a definitive treatment for GOO. However, it leads to operative morbidity, longer hospital stay, as well as more complications [3, 12]. Some causes of benign GOO may be reversible, surgical gastroenterostomy and permanent insertion of SEMS may not be necessary [11]. Additionally, surgical treatment is more invasive, meaning many patients are not good candidates due to age or comorbidities.
Benign GOO is usually treated with balloon dilation, and most patients with benign GOO respond well to balloon dilation; however, repeated dilation sessions are required to achieve clinical success. Benign GOO caused by extrinsic compression, such as chronic pancreatitis, may be resistant to balloon dilation [2]. Early studies have shown that endoscopic balloon dilation has a high rate of failure for treating peptic GOO, and surgical treatment is required in most patients. Boylan et al. reported that after balloon dilation in 40 patients with benign GOO, obstruction symptoms relieved in 30% of patients, and recurrence of obstruction was observed in the remaining 70% patients; ultimately, about 30% of the patient required surgical treatment [13]. Cherian et al. [14] speculated that treatment failure was likely related to the fact that no systematical treatment was conducted in most of these studies to manage the underlying peptic ulcer disease. They reviewed 23 consecutive patients with peptic GOO who were treated with balloon dilation and medical therapy, a favorable long-term outcome was observed despite its small sample size [14].
Compared with surgical gastrojejunostomy, fully covered SEMS placement is an elective procedure for the management of GOO, which increases the number of candidates, shortens hospital stay, reduces healthcare costs, and has decreased complications [15]. Jeurknink et al. reported that stenting procedure has a higher clinical success rate in improving oral intake and relieving symptoms in patients with malignant GOO compared with surgical gastrojejunostomy [16]. Randhawa et al. reported that symptoms were relieved immediately after insertion of fully covered SEMS, and all patients were able to tolerate a soft diet on the first day after procedure [1]. However, only 28 patients presenting with different kinds of benign strictures (e.g., the pylorus, duodenum, or gastrojejunal anastomosis) were entered into their study. In our study, we provide a larger series to date comparing these approaches and focus on benign GOO rather than benign upper gastrointestinal stricture. In addition, it is the first study from our region to include cost analysis and long-term follow-up for benign GOO.
SEMS placement may not be a good option for benign GOO as there are no suitable stents [11]. Uncovered stents may become difficult to be removed after long-term insertion, leading to restenosis of stent and reduced patency rate. In our study, 9 patients had a recurrence of obstructive symptoms due to stent-related complications, including stent restenosis in 7 patients and stent fracture or obstruction in 2 patients, respectively. Although covered SEMS may reduce the risk of restenosis, an obvious drawback is stent migration from the site of insertion [17]. For example, in a prospective study with 28 patients [1], the immediate complication of stent migration was noted in eight patients, with a migration rate as high as 28.6%. SEMS migration occurred in 7 patients in our study. Six patients had distal migration while one had proximal migration into the stomach. Thus, the long-term efficacy and safety of SEMS is uncertain, with high rates of reintervention, hospital stay, and healthcare costs.
Currently, various techniques of anti-migration have been developed to overcome stent migration. Among these, endoscopic clipping has proven effective in decreasing the migration rate [18]. For example, Randhawa et al. [1] reported that using an Endostich device to suture and fix the stent can provide better fixation, reducing the risk of stent migration. In the study of Randhawa et al. [1], a single suture was used to secure in the initial eight patients and a high rate of stent migration was documented; two to three sutures were used to strengthen the fixation in the rest of the patients, and no distal stent migration was found.
The limitations of this study include a single-center retrospective design with relatively small sample size; a lack of a comparison study with EUS-GJ or surgical gastrojejunostomy; the use of uncovered SEMS for treating benign GOO is controversial, and long-term outcomes remain to be observed. Future studies with large sample size are wanted and comparison studies with surgical gastrojejunostomy and/or EUS-GJ should be conducted to further validate our findings. Nevertheless, in this context, a series of 70 patients (with subgroup analysis by etiology) provides valuable real-world data. The inclusion of cost analysis and detailed breakdown of complications (like stent migration, restenosis rates, etc.) is a useful addition to the literature. We also stratified outcomes for post-surgical strictures versus other benign causes, which offers a novel nuance, and this kind of subgroup comparison may help tailor treatment decisions based on stricture etiology.