The 2025 chikungunya outbreak has surged from the Indian Ocean to Europe, prompting an urgent global research response. With no antivirals and limited vaccine access, laboratories and biotech firms are under pressure to deliver solutions fast.

The world is facing the largest recorded global spread of chikungunya virus (CHIKV), with outbreaks this year reported in more than 119 countries and extending into temperate zones of Europe. The mosquito-borne virus, long regarded as a tropical health concern, has surged on the Indian Ocean islands of Réunion, Mayotte and Mauritius. Autochthonous (locally acquired) transmission has also been confirmed in France’s Grand Est and south-eastern regions. The World Health Organization (WHO) estimates that 5.6 billion people may be at risk of exposure in 2025.
Expanding threat
Chikungunya belongs to the alphavirus genus and is primarily transmitted by Aedes aegypti and Aedes albopictus mosquitoes. Symptoms typically include sudden onset of high fever, rash and severe joint pain, often accompanied by muscle aches and headache. Although the disease is rarely fatal, it can leave patients with chronic pain and disability lasting months or years. Vulnerable groups such as infants, older adults and those with underlying conditions are particularly affected.
Chikungunya belongs to the alphavirus genus and is primarily transmitted by Aedes aegypti and Aedes albopictus mosquitoes.
This year’s expansion of transmission is being attributed to a convergence of factors. Climate change has enabled Aedes mosquitoes to colonise new regions, while rapid urbanisation provides ideal breeding environments. International travel has accelerated the spread of the virus to non-endemic regions and the absence of herd immunity following several years without large-scale outbreaks has left populations exposed. Surveillance challenges and inconsistent access to diagnostics have further delayed early detection, allowing the virus to establish footholds in new areas.
A recent Lancet commentary described chikungunya’s return to Europe as “a turning point for the global arboviral landscape”, according to Zumla et al. published earlier this year. The World Health Organization has also raised concern over the pace of spread, warning in its June 2025 update that the virus has reached regions not previously considered at risk.
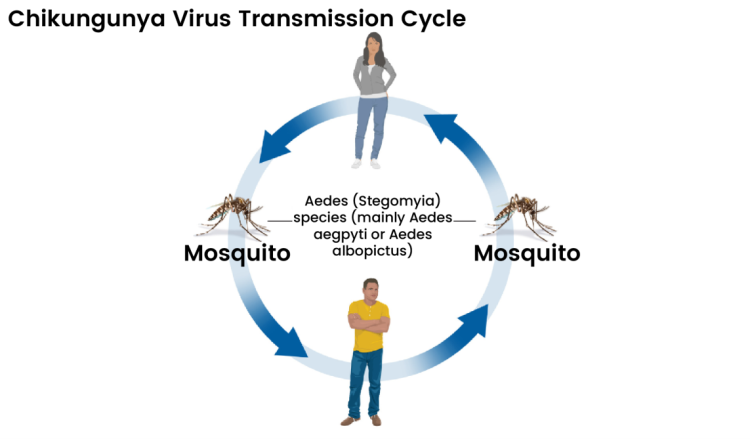

The transmission cycle of chikungunya virus. The virus spreads primarily via Aedes aegypti and Aedes albopictus mosquitoes. Infected mosquitoes pass the virus to humans through bites, while infected humans can in turn infect new mosquitoes, sustaining the cycle. Image credit: CDC
Limited treatment options
There is still no licensed antiviral treatment for CHIKV. Clinical management remains supportive, focused on relieving fever and inflammation to reduce patient discomfort. This therapeutic gap is particularly problematic in low-resource settings where health systems are already burdened by dengue and malaria.
The vaccine pipeline, however, has begun to bear fruit. Two candidates have reached the market in recent years:
- IXCHIQ, a live-attenuated vaccine, is approved for adults, though with restrictions for older populations due to safety considerations.
- VIMKUNYA, a virus-like particle vaccine, is authorised as a single-dose injection for individuals aged 12 and above.
While these vaccines mark important milestones, global access remains limited. Regulatory timelines, cost considerations and supply constraints continue to leave much of the world reliant on vector control, community awareness campaigns and case surveillance as the primary tools of defence.
Research response gains momentum
The scale of the 2025 outbreak has galvanised both academic and industry-led research. Efforts include testing vaccine durability and cross-protection against emerging CHIKV variants. Researchers are also screening compound libraries for antiviral activity, while new diagnostics are being designed to distinguish chikungunya from co-circulating viruses such as dengue and Zika.
Laboratory reagents have proven central to this accelerated response. Recombinant viral proteins and antibodies are allowing researchers to model immune responses, evaluate neutralising titres and design next-generation diagnostic assays.
To address the ongoing CHIKV outbreak, Sino Biological swiftly initiated the development of recombinant E1 and E2 proteins, together with specific antibodies. These reagents are providing crucial support for researchers working on vaccine development, antiviral therapies and immunodiagnostic assay creation.
The company also supplies key tools including the recombinant CHIKV E2 glycoprotein, complementary DNA (cDNA) clones and specialised antibodies for enzyme-linked immunosorbent assay (ELISA) and lateral flow assays (LFA). All are produced under strict quality controls to ensure reliability.
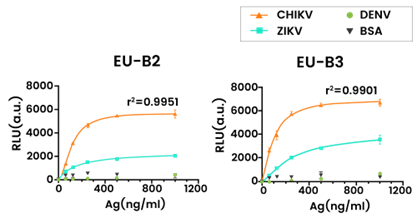
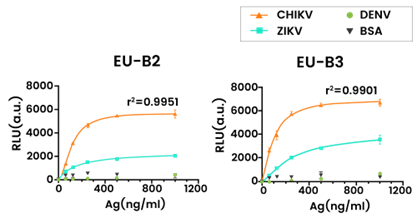
Detection of recombinant CHIKV-E2, ZIKV-E and DENV-E proteins at serial dilutions using peptide aptamers B2 and B3. Image credit: Liu et al. 2023
A number of peer-reviewed publications have employed Sino Biological proteins and antibodies. Examples include:
- Liu and Gu employed the recombinant CHIKV E2 protein (40440-V08B) in an aptamer-based time-resolved fluoroimmunoassay, demonstrating peptide binding affinity and specificity to E2 antigens.
- Tamburini et al. investigated viral antigen acquisition in lymphatic endothelial cells (LEC), blood endothelial cells (BEC) and fibroblastic reticular cells (FRC) in mice infected with recombinant CHIKV E2 conjugated to AlexaFluor-488. They reported that both LECs and a subset of FRCs acquired the viral protein during the treatment window.
- Kim et al. used recombinant E2 protein pre-coated ELISA plates to compare immune responses, showing that glycan-mutant envelope proteins induced lower humoral responses than the full-length E protein.
By supplying validated reagents for these investigations, Sino Biological has enabled scientists to accelerate discovery at a time when speed and reliability are crucial.
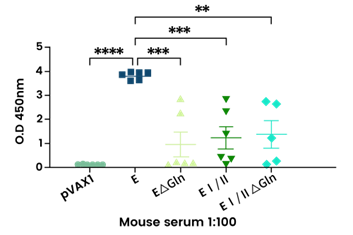
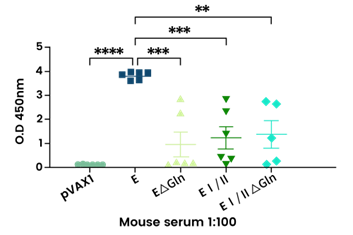
Measurement of total IgG levels against CHIKV-E2 in sera from individual mice using ELISA with recombinant CHIKV-E2 protein pre-coated plates. Image credit: Kim et al. 2024
Industry and academic partnerships
The outbreak has also highlighted the growing importance of collaboration between biotechnology companies and academic researchers. Reliable reagents shorten experimental timelines and reduce variability, accelerating the transition from early discovery to translational research. As chikungunya spreads into regions previously unprepared for arboviral outbreaks, the ability to generate reproducible, high-quality data quickly has become a critical part of the response.
From outbreak to action
The chikungunya outbreak of 2025 is a reminder that arboviruses do not respect geographical boundaries. As WHO and national health authorities work to strengthen vector control and surveillance, researchers are racing to fill the therapeutic and diagnostic gaps.
For drug discovery scientists, the outbreak represents both a challenge and an opportunity. The urgent need for antivirals, widely available vaccines and scalable diagnostics is driving innovation across multiple fronts. Partnerships between public health agencies, academic groups and industry providers such as Sino Biological are proving essential to this effort. Further details of Sino Biological’s portfolio of recombinant antigens and antibodies for chikungunya research are available on the company’s website.
The global health community has learned difficult lessons from dengue and Zika. Chikungunya is now firmly established as a worldwide threat, making it a priority to translate scientific advances into accessible interventions before the next epidemic wave arrives.
References
- Adam A, et al. A safe insect-based chikungunya fever vaccine affords rapid and durable protection in cynomolgus macaques. NPJ Vaccines. 2024;9:65.
- Centers for Disease Control and Prevention. Areas at risk for chikungunya [Internet]. Atlanta (GA): CDC; 2025 [cited 2025 Aug 22]. Available from: https://www.cdc.gov/chikungunya/data-maps/index.html
- de Souza WM, Lecuit M, Weaver SC. Chikungunya virus and other emerging arthritogenic alphaviruses. Nat Rev Microbiol. 2025;23(7):401–15. doi:10.1038/s41579-025-01177-8.
- Doan TA, et al. Immunization-induced antigen archiving enhances local memory CD8+ T cell responses following an unrelated viral infection. NPJ Vaccines. 2024;9:112.
- European Centre for Disease Prevention and Control. Chikungunya virus disease worldwide overview. Stockholm: ECDC; 2025. Preprint.
- Frumence E, et al. Genomic insights into the re-emergence of chikungunya virus on Réunion Island, France, 2024 to 2025. Euro Surveill. 2025;30(12):240032.
- Kim K, et al. Immunogenicity analysis of chikungunya virus DNA vaccine based on mutated putative N-linked glycosylation sites of the envelope protein. Vaccines (Basel). 2024;12(5):842.
- Liu T, et al. Peptide aptamer-based time-resolved fluoroimmunoassay for CHIKV diagnosis. Virol J. 2023;20:189.
- Roiz D, Boussès P, Simard F, Paupy C, Fontenille D. Autochthonous Chikungunya transmission and extreme climate events in Southern France. PLoS Negl Trop Dis. 2015;9(6):e0003854.
- World Health Organization. Chikungunya epidemiology update – June 2025 [Internet]. Geneva: WHO; 2025 [cited 2025 Aug 22]. Available from: https://www.who.int/publications/m/item/chikungunya-epidemiology-update-june-2025
- World Health Organization raises concern about spread of mosquito-borne Chikungunya virus. Reuters [Internet]. 2025 Jul 22 [cited 2025 Aug 22]. Available from: https://www.reuters.com/business/healthcare-pharmaceuticals/world-health-organization-raises-concern-about-spread-mosquito-borne-chikungunya-2025-07-22/
- What is the chikungunya virus, how are countries such as China battling it? Al Jazeera [Internet]. 2025 Aug 6 [cited 2025 Aug 22]. Available from: https://www.aljazeera.com/news/2025/8/6/what-is-the-chikungunya-virus-how-are-countries-such-as-china-battling-it
- Zumla A, Ntoumi F, Ippolito G, PANDORA-ID-NET Consortium. Chikungunya virus disease returns to Europe: a turning point for the global arboviral landscape. Lancet. 2025;405(10332):345–7.