In this population-based cohort study of Iranian women, we investigated the association between quartiles of various heavy metals and serum AMH levels. Our results suggest that increased Cu exposure may lead to decreased AMH levels.
Heavy metals have long been recognized as toxic, and clinical consequences that vary widely. Being non-degradable, they can adversely affect health through ingestion, inhalation, or dermal contact. These metals can bind to vital enzymes or replace other elements in biochemical reactions, leading to toxic effects [10]. There is evidence that some heavy metals are harmful to reproductive health [24,25,26,27].
Numerous experimental studies suggest several plausible mechanisms by which Cu may influence ovarian reserve. Fluctuations in Cu levels have been shown to impact the functions of neutrophils, monocytes, and lymphocytes within the immune system [20]. Elevated Cu levels may contribute to increased oxidative stress, inflammation, and immune disruption, which can damage cellular structures and impair normal ovarian function. This oxidative stress may lead to follicular atresia, reduced estrogen production, and ultimately premature ovarian insufficiency. For exampple, Kebapcilar et al. [20] conducted a study in Turkey showing that women with premature ovarian failure had higher blood serum Cu concentration compared to women with normal menstrual cycle. Experimental studies have also demonstrated that Cu can induce apoptosis in ovarian cells through pathways involving mitochondrial dysfunction and activation of apoptotic proteins, such as caspase 3, via the MAPK14-Nrf2 signaling pathway [28,29,30]. Additionally, Cu may interact with the hypothalamic-pituitary-gonadal axis, influencing the secretion of reproductive hormones and further impacting ovarian reserve [31, 32].
In our study, no significant association was found between Pb exposure and AMH levels. This aligns with findings from Christensen et al. [22], who reported no significant association between plasma Pb levels and AMH in women with primary ovarian failure. Similarly, Wafa et al. [33] in Egypt, found no significant association between plasma Pb levels and AMH in this population. Paksy et al. [34] also reported that Pb levels in the ovarian follicle fluid did not significantly affect progesterone secretion, an indicator of ovarian reserve. Furthermore, another study examining serum AMH and urinary Pb levels found no association between elevated Pb concentrations and decreased AMH [35].
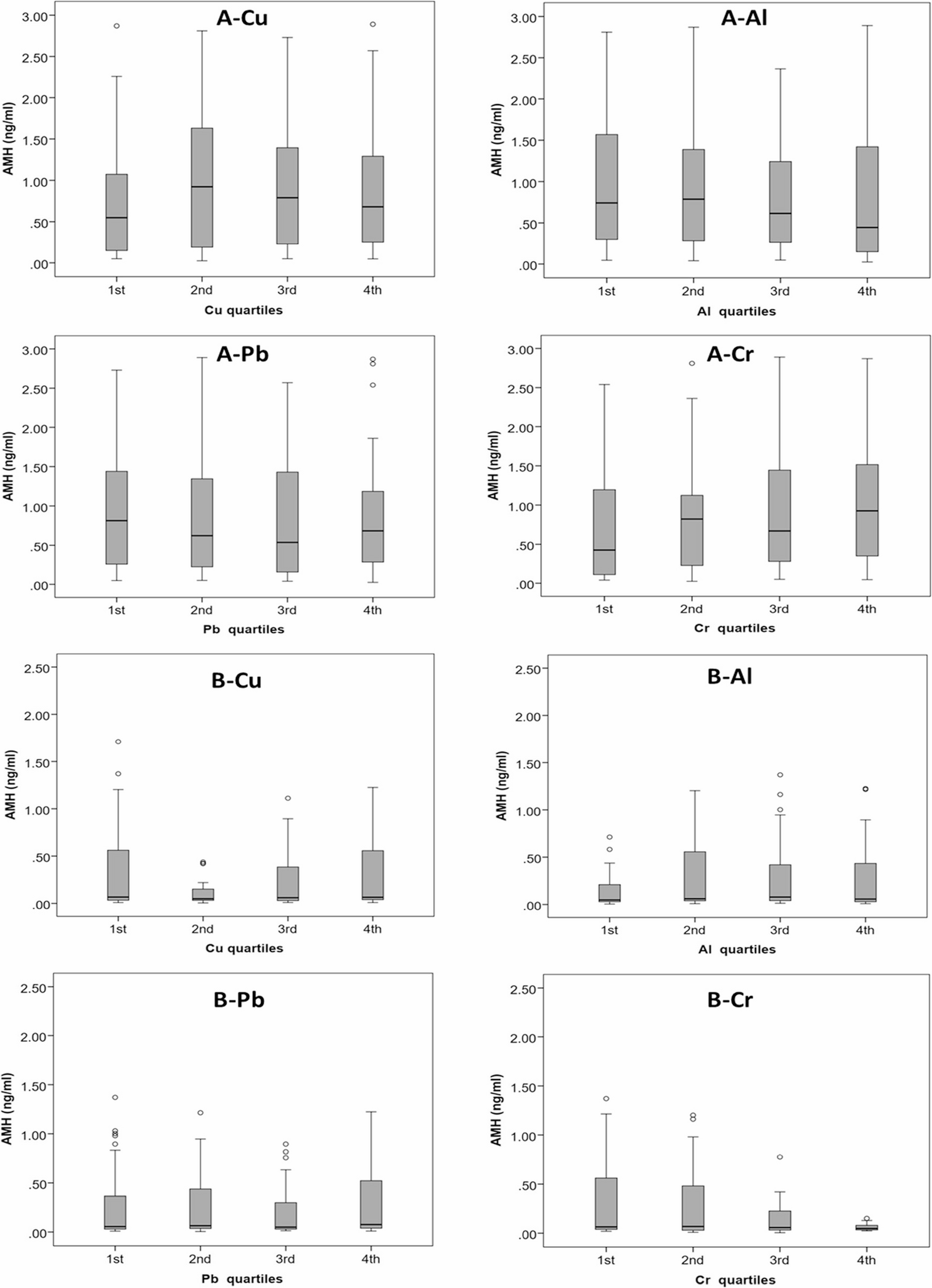
Box and whisker plots of the distributions of Cu, Al, Pb, and Cr in the samples from the A 2nd and B 5th follow-up visits
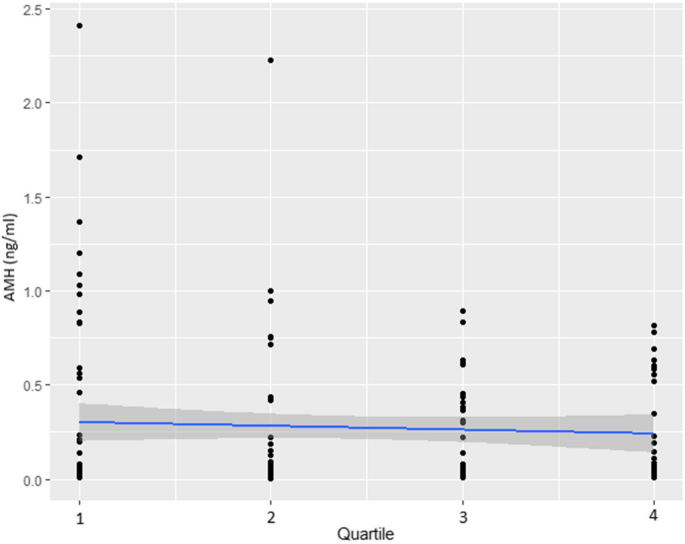
Trend of AMH (ng/ml) by quartiles of Cu at 5th follow-up
Al is a ubiquitous in the environment [36], with the primary route of exposure being through the diet, particularly as food additives [37]. In our study, no statistically significant relationship was observed between Al exposure and AMH levels. Similarly, Ozal et al. [10] in Turkey reported higher serum Al levels in women with primary ovarian failure compared to controls, but the difference was not statistically significant.
Regarding Cr, some studies have reported significant associations between Cr exposure and diminished ovarian reserve, as well as potential benefits of Cr supplementation in improving metabolic dysfunction and lipid and carbohydrate metabolism in women with polycystic ovarian syndrome.
Although Cr (VI) and Cr (0) are commonly produced by industrial processes and can cause tissue damage by inducing oxidative stress and cellular damage. Hexavalent Cr leads to increased formation of reactive oxygen species (ROS) such as superoxide anions, hydroxyl radical and nitric oxide, decreased cell viability, increased cellular genomic DNA fragmentation, membrane damage, apoptotic cell death and necrosis [38]. Our study found no significant association between Cr concentration and AMH levels.
In our study, Cd concentration in serum samples were below the detection limit, preventing meaningful analysis. This contrasts with findings of Christensen et al. [22], who observed a positive correlation between Cd and serum AMH. Lee et al. [19] in South Korea reported that environmental Cd exposure (mean 0.97 µg/l) was associated with decreased AMH levels (mean 3.2 ng/ml) in premenopausal women aged 30 and 35. The discrepancy in the relationship between Cr concentration and AMH levels may be partly attributed to the differences in the age of the participants. In our study, the participants were older (42 and 51 years old at the 2nd and 5th visits) and had lower AMH values (0.68 and 0.06 ng/ml) compared to the participants in Lee et al.‘s study (30–35 years old, AMH 0.97 ng/ml).
Variations findings across studies may stem from differences in environmental or occupational exposure levels, age distribution, reproductive status, health conditions, biological matrices used (serum vs. urine), and timing of sample collection [39,40,41]. Some evidence suggests that the association between Cd and AMH may be more pronounced in specific age groups, such as women aged 30–35 [19]. In our study, the lack of significant associations with Cd or Pb may reflect lower exposure levels, differences in participant characteristics, or the specific biomarkers and methods used.
We implemented rigorous measures, including standardized AMH assays, well-defined inclusion/exclusion criteria to control for factors affecting ovarian reserve, and multivariate adjustments for confounders, to minimize bias. However, unmeasured confounders—such as occupational heavy metal exposure, endocrine-disrupting chemicals, other environmental pollutants, and dietary factors—were not accounted for and could influence both heavy metal and AMH levels.
Regarding the strengths of this study, it is based on a well-established population-based cohort, minimizing intra-assay and inter-assay variability in AMH measurement since as all assays were conducted in the same laboratory by an experienced technician. Furthermore, data were collected at two time points over a 10-year follow-up period within this cohort, enhancing the study’s longitudinal rigor. Rigorous inclusion and exclusion criteria were applied to ensure that AMH measurements were not be confounded by conditions or interventions known to affect hormonal balance or the ovarian aging. This approach strengthens the validity of our findings on the relationship between heavy metal exposure and ovarian reserve. Additionally, statistical adjustments were made to control for potential confounders, ensuring the robustness of the results. Although a slight decrease in AMH levels was observed at higher Cu quartiles, the absence of statistical significance in the trend analysis calls for cautious interpretation.
The present study is not without its limitations. While our findings suggest an association between higher Cu levels and lower AMH, causality cannot be definitively established due to the observational nature of our research. While repeated samples were indeed available at the second and fifth follow-ups, both heavy metal and AMH measurements were collected concurrently at same time, reflecting simultaneous exposure and outcome assessments. Given the potential for short-term fluctuations in metal concentrations and hormone levels, we prioritized cross-sectional analyses to more accurately characterize these concurrent associations. Despite our efforts to adjust for a comprehensive set of known confounders in the multivariate analyses, we acknowledge that unmeasured factors, such as exposure to endocrine-disrupting chemicals and other environmental pollutants, may have influenced the observed associations. The dataset did not include detailed data on dietary intake, occupational exposure, and other environmental sources. It is recommended that subsequent studies take these factors into consideration to more effectively control for confounding variables. We excluded smokers from our study due to the well-established impact of smoking on ovarian reserve, as measured by AMH levels. Multiple studies have shown that smoking significantly reduces AMH levels, indicating diminished ovarian reserve [42,43,44]. This reduction is likely caused by toxic substances in cigarette smoke, which induce oxidative stress and damage ovarian follicular cells [45]. Furthermore, smoking disrupts endocrine function, altering AMH and other reproductive hormone levels [46]. Including smokers in our study could introduce confounding variability unrelated to the primary factors being investigated, thus affecting the accuracy of AMH as an indicator of ovarian reserve. However, this exclusion limits the generalizability of our findings to non-smoking populations. Our study population is drawn exclusively from the TLGS cohort, which may limit the external validity due to specific cultural, environmental, and genetic characteristics unique to this group.
Another limitation is the relatively advanced age of participants (mean of 42 and 51 years at two follow-ups), which may obscure the observed subtle effects of heavy metals on ovarian reserve, particularly in younger women. Additionally, AMH was the sole ovarian reserve marker used; inclusion of complementary markers such as antral follicle count (AFC) or FSH levels could provide a more comprehensive assessment. we did not employed the most sensitive assay for AMH measurement (pico AMH), which detects very low ovarian reserves [47], though Gen II and pico AMH assays correlate highly [48]. Samples were stored and not collected on specific menstrual cycle days; However, evidence suggests that AMH values remain stable during long-term storage and is unaffected by menstrual cycle [49]. Serum Cu levels may not fully capture long-term or cumulative exposure compared to urinary measurements.
Several factors may explain the of lack of significant association between AMH and Pb, Cd, Al, and Cr: the modest sample size and geographic restriction to a specific Tehran population may have limited exposure variability. Such homogeneity in environmental and lifestyle factors can constrain the ability to detect significant associations. Exposure measurement error is a recognized challenge in environmental epidemiology and may attenuate the observed associations, particularly when exposures are low or fluctuate over time. Although we employed validated bio monitoring methods, some degree of measurement imprecision is inevitable and could have limited our statistical power to detect subtle effects. Also the sample size and statistical power may have been insufficient to reveal associations for metals with lower exposures or weaker effects on ovarian reserve. We also did not explicitly model interactions or assess multicollinearity among the metals. These issues could affect the observed associations and may mask or exaggerate the effects of individual metals.