Study finds that lithium loss triggers Alzheimer’s, but a lithium-based compound reverses the disease in mice.
What sets off the earliest chain of events that leads to Alzheimer’s disease? And why do some people who show Alzheimer’s-like brain changes never progress to dementia? These unresolved questions have challenged scientists for decades.
Researchers at Harvard Medical School now believe they may have an explanation: a deficiency of lithium in the brain.
Their study, published on August 6 in Nature, demonstrates for the first time that lithium is naturally present in the brain, where it protects against neurodegeneration and supports the normal functioning of all major brain cell types. The findings — a decade in the making — draw on mouse experiments as well as analyses of human brain tissue and blood samples from people at different stages of cognitive health.
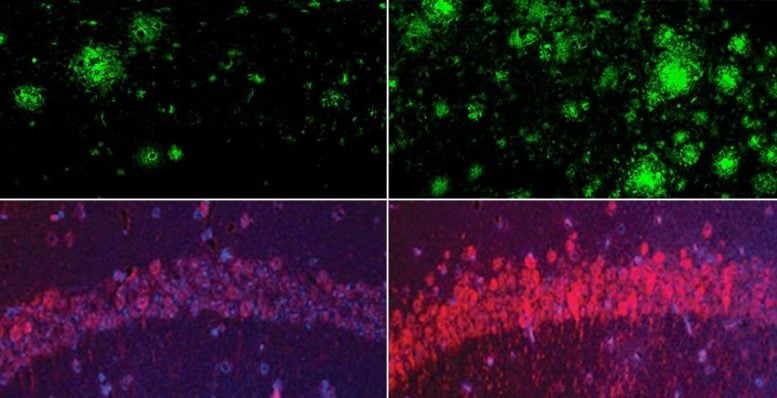
The team discovered that loss of lithium in the human brain is among the earliest alterations linked to Alzheimer’s, while in mice, reduced lithium sped up both brain damage and memory decline. They traced this decline to lithium binding with amyloid plaques and reduced absorption in the brain. In follow-up experiments, they showed that a new lithium compound resistant to amyloid capture restored memory in mice.
Together, the results bring coherence to decades of patient observations and introduce a new framework for understanding the disease, offering potential paths for early diagnosis, prevention, and treatment.
Filling gaps in Alzheimer’s research
Alzheimer’s disease, which affects an estimated 400 million people worldwide, is marked by a range of brain abnormalities. These include clumps of the protein amyloid beta, tangles of the protein tau, and the loss of a protective protein called REST. Yet none of these features fully account for the disease. Some individuals with such changes show no cognitive impairment, and drugs designed to target amyloid beta often fail to restore memory and provide only limited slowing of decline.
It is also well established that both genetic and environmental influences shape a person’s risk, but researchers have long struggled to explain why people with the same risk factors can experience such different outcomes.
The new study suggests that lithium may represent a missing link.
“The idea that lithium deficiency could be a cause of Alzheimer’s disease is new and suggests a different therapeutic approach,” said senior author Bruce Yankner, professor of genetics and neurology in the Blavatnik Institute at HMS, who in the 1990s was the first to demonstrate that amyloid beta is toxic.
According to Yankner, the findings open the possibility that lithium could eventually be used to treat the disease as a whole rather than targeting only one aspect, such as amyloid beta or tau.
Amyloid beta binds lithium
A key finding from the study is that during the early stages of dementia, amyloid beta begins forming deposits that bind to lithium in both humans and mouse models. This binding reduces lithium’s availability in the brain, lowering its function across all major brain cell types. In mice, the drop in lithium levels triggered changes that mirrored Alzheimer’s disease, including memory impairment.
The researchers identified a group of lithium compounds capable of avoiding capture by amyloid beta. Among them, lithium orotate proved the most effective: in mice, it reversed Alzheimer’s-like pathology, protected brain cells from damage, and restored memory function.
While these results must still be validated in humans through clinical trials, they suggest that monitoring lithium levels could help detect Alzheimer’s at an early stage. They also highlight the potential of amyloid-resistant lithium compounds as candidates for treatment or prevention.
Lithium-based drugs are already prescribed for conditions such as bipolar disorder and major depression, but those therapies require doses high enough to be toxic, particularly in older adults. In contrast, Yankner’s team showed that lithium orotate works at a concentration about one-thousandth of the clinical dose — enough to replicate natural brain lithium levels. Mice given this compound for nearly their entire lifespan showed no signs of toxicity.
“You have to be careful about extrapolating from mouse models, and you never know until you try it in a controlled human clinical trial,” Yankner said. “But so far the results are very encouraging.”
Lithium depletion is an early sign of Alzheimer’s
Yankner’s interest in lithium began while studying its relationship to REST, a protein known for its neuroprotective role. Determining whether lithium naturally exists in the human brain, and whether its levels shift during the progression of neurodegeneration, required access to brain tissue — something not possible to obtain from living individuals.
To address this, the lab partnered with the Rush Memory and Aging Project in Chicago, which maintains a collection of postmortem brain samples donated by thousands of participants spanning the full spectrum of cognitive health and decline.
This breadth of material was essential. Studying advanced Alzheimer’s is, as Yankner described it, “like looking at a battlefield after a war,” where extensive damage obscures the origins of the disease. In contrast, examining early stages allows researchers to find “important clues before the brain is badly damaged.”
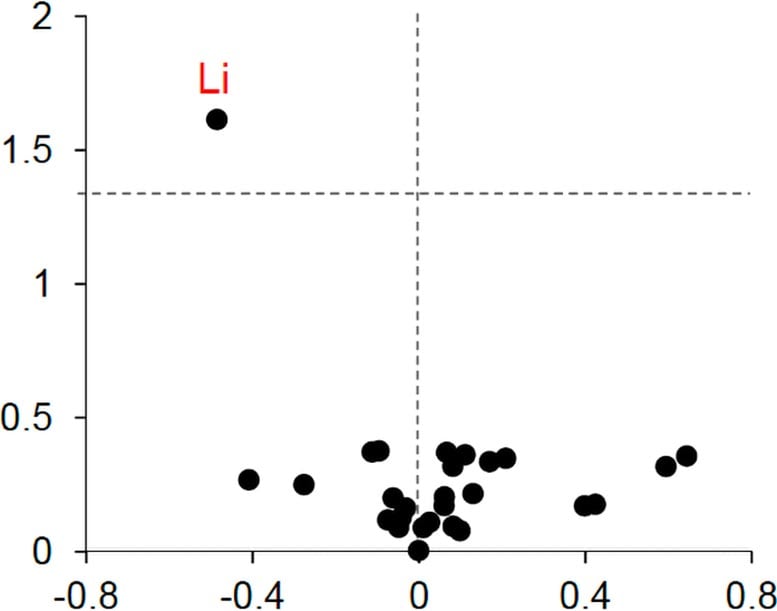
Under the leadership of first author Liviu Aron, senior research associate in the Yankner Lab, the team used advanced mass spectroscopy to measure trace levels of about 30 metals in brain and blood samples from individuals who were cognitively healthy, those with mild cognitive impairment, and those with advanced Alzheimer’s.
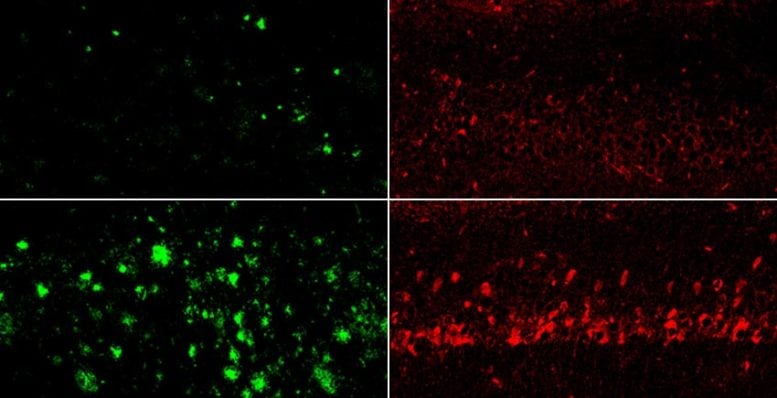
Lithium was the only metal that showed clear differences among groups and declined at the earliest stages of memory loss. Healthy donors had higher lithium levels, while those with mild impairment or full Alzheimer’s exhibited substantially reduced levels.
The team confirmed these results with additional samples from brain banks across the United States.
This observation aligned with earlier population studies that linked higher environmental lithium — such as lithium naturally occurring in drinking water — with lower rates of dementia.
Crucially, however, the new research went further by directly detecting lithium in the brains of individuals who had never received it as a therapy, establishing a normal physiological range and showing that lithium is vital for brain function.
“Lithium turns out to be like other nutrients we get from the environment, such as iron and vitamin C,” Yankner said. “It’s the first time anyone’s shown that lithium exists at a natural level that’s biologically meaningful without giving it as a drug.”
Then Yankner and colleagues took things a step further. They demonstrated in mice that lithium depletion isn’t merely linked to Alzheimer’s disease — it helps drive it.
Loss of lithium causes the range of Alzheimer’s-related changes
The researchers found that feeding healthy mice a lithium-restricted diet brought their brain lithium levels down to a level similar to that in patients with Alzheimer’s disease. This appeared to accelerate the aging process, giving rise to brain inflammation, loss of synaptic connections between neurons, and cognitive decline.
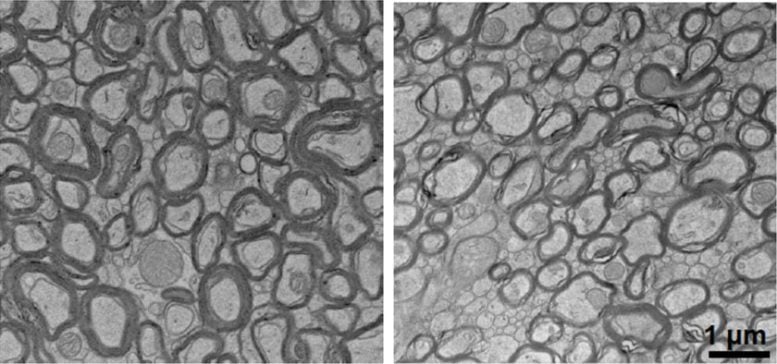
In Alzheimer’s mouse models, depleted lithium dramatically accelerated the formation of amyloid-beta plaques and structures that resemble neurofibrillary tangles. Lithium depletion also activated inflammatory cells in the brain called microglia, impairing their ability to degrade amyloid; caused the loss of synapses, axons, and neuron-protecting myelin; and accelerated cognitive decline and memory loss — all hallmarks of Alzheimer’s disease.
The mouse experiments further revealed that lithium altered the activity of genes known to raise or lower risk of Alzheimer’s, including the most well-known, APOE.
Replenishing lithium by giving the mice lithium orotate in their water reversed the disease-related damage and restored memory function, even in older mice with advanced disease. Notably, maintaining stable lithium levels in early life prevented Alzheimer’s onset — a finding that confirmed that lithium fuels the disease process.
“What impresses me the most about lithium is the widespread effect it has on the various manifestations of Alzheimer’s. I really have not seen anything quite like it all my years of working on this disease,” said Yankner.
A promising avenue for Alzheimer’s treatment
A few limited clinical trials of lithium for Alzheimer’s disease have shown some efficacy, but the lithium compounds they used — such as the clinical standard, lithium carbonate — can be toxic to aging people at the high doses normally used in the clinic.
The new research explains why: Amyloid beta was sequestering these other lithium compounds before they could work. Yankner and colleagues found lithium orotate by developing a screening platform that searches a library of compounds for those that might bypass amyloid beta. Other researchers can now use the platform to seek additional amyloid-evading lithium compounds that might be even more effective.
“One of the most galvanizing findings for us was that there were profound effects at this exquisitely low dose,” Yankner said.
If replicated in further studies, the researchers say lithium screening through routine blood tests may one day may offer a way to identify individuals at risk for Alzheimer’s who would benefit from treatment to prevent or delay disease onset.
Studying lithium levels in people who are resistant to Alzheimer’s as they age might help scientists establish a target level that they could help patients maintain to prevent onset of the disease, Yankner said.
Since lithium has not yet been shown to be safe or effective in protecting against neurodegeneration in humans, Yankner emphasizes that people should not take lithium compounds on their own. But he expressed cautious optimism that lithium orotate or a similar compound will move forward into clinical trials in the near future and could ultimately change the story of Alzheimer’s treatment.
“My hope is that lithium will do something more fundamental than anti-amyloid or anti-tau therapies, not just lessening but reversing cognitive decline and improving patients’ lives,” he said.
Reference: “Lithium deficiency and the onset of Alzheimer’s disease” by Liviu Aron, Zhen Kai Ngian, Chenxi Qiu, Jaejoon Choi, Marianna Liang, Derek M. Drake, Sara E. Hamplova, Ella K. Lacey, Perle Roche, Monlan Yuan, Saba S. Hazaveh, Eunjung A. Lee, David A. Bennett and Bruce A. Yankner, 6 August 2025, Nature.
DOI: 10.1038/s41586-025-09335-x
This work was supported by the National Institutes of Health (grants R01AG046174, R01AG069042, K01AG051791, DP2AG072437, P30AG10161, P30AG72975, R01AG15819, R01AG17917, U01AG46152, and U01AG61356), the Ludwig Family Foundation, the Glenn Foundation for Medical Research, and the Aging Mind Foundation.
Never miss a breakthrough: Join the SciTechDaily newsletter.