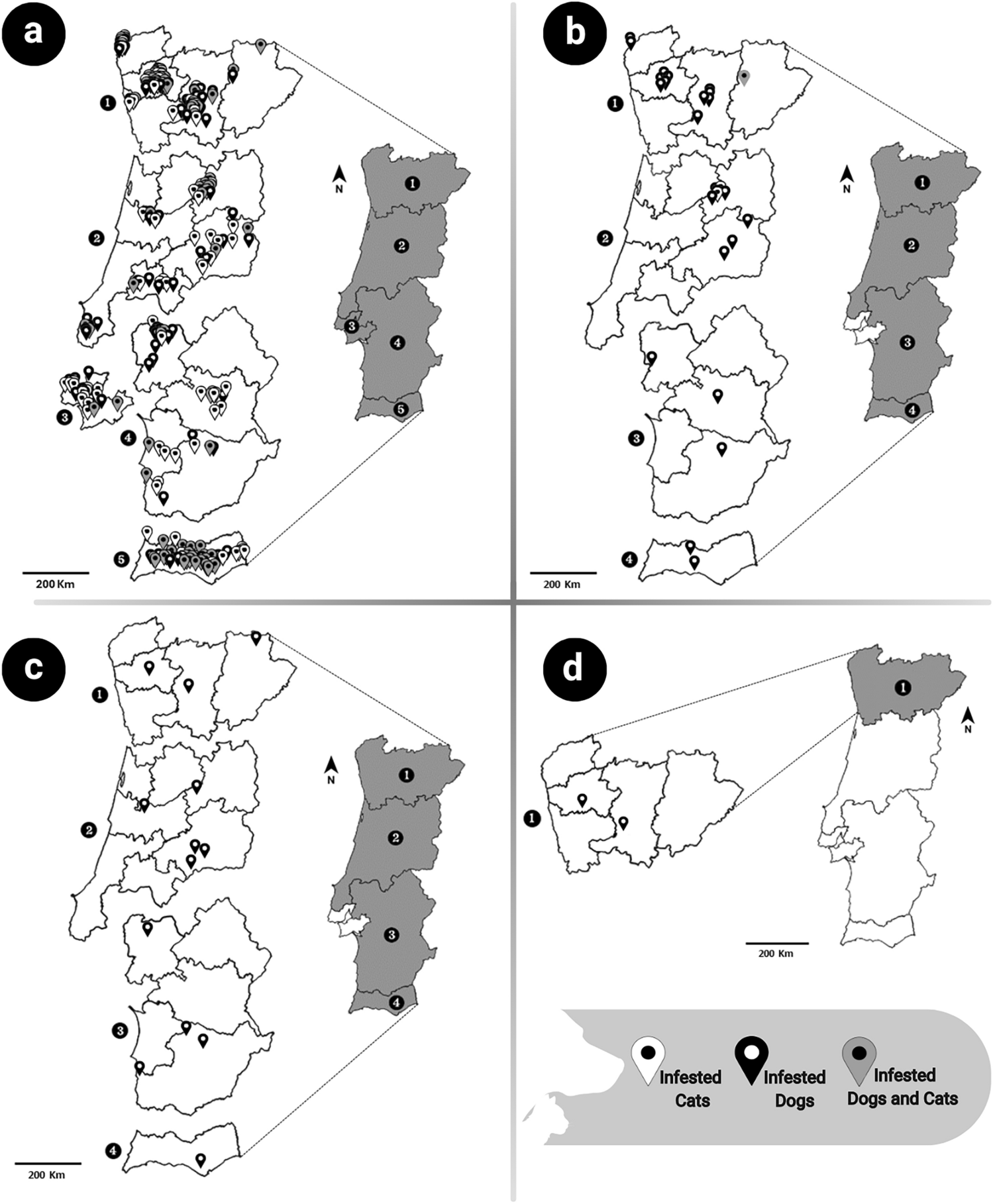
Flea infestation prevalence and patterns
A total of 1052 dogs and 1039 cats from 94 counties (64 rural and 43 non-rural) were examined, with an overall flea infestation prevalence of 33.6% (353/1052) in dogs and 36.5% (379/1039) in cats (Tables 1, 2). No statistically significant difference was observed between dogs and cats in terms of infestation prevalence (χ2 = 2.05, df = 1, P = 0.153).
The prevalence of flea infestation did not differ significantly between sexes in either species (dogs: χ2 = 0.36, df = 1, P = 0.550; cats: χ2 = 0.16, df = 1, P = 0.691). In dogs, 32.2% of females (151/469) and 34.0% of males (166/488) were infested. In cats, infestation was recorded in 36.4% of females (177/486) and 35.2% of males (170/483).
A significant association was observed between flea infestation and age group in both dogs (P = 0.015) and cats (χ2 = 21.46, df = 5, P = 0.001). Puppies (age < 6 months; 45.8%, 44/96, ASR = 2.8) and kittens (age ≤ 6 months; 50.0%, 67/134, ASR = 3.6) exhibited the highest prevalence of infestation.
In dogs, fur length was significantly associated with infestation (χ2 = 14.95, df = 2, P = 0.001), with short-haired individuals presenting the lowest prevalence of infestation (28.0%, 151/540, ASR = − 3.8).
Lower body condition scores were significantly associated with higher flea infestation prevalence in both dogs (42.0%, 66/157, χ2 = 7.98, df = 2, P = 0.019, ASR = 2.5) and cats (44.2%, 88/199, χ2 = 11.41, df = 2, P = 0.003, ASR = 2.6).
Geographical origin also influenced flea infestation prevalence, with the highest values recorded in the North (dogs: 45.1%, 143/317, χ2 = 69.74, df = 4, P < 0.0001, ASR = 5.3; cats: 45.0%, 139/309, χ2 = 31.67, df = 4, P < 0.0001, ASR = 3.6) and the lowest recorded in the LMA (dogs: 9.9%, 19/192, ASR = − 7.6; cats: 20.0%, 37/185, ASR = − 5.2). In contrast, no significant differences were found regarding parish type (dogs: χ2 = 0.09, df = 1, P = 0.765; cats: χ2 = 0.75, df = 1, P = 0.387).
Lifestyle was significantly associated with flea infestation (dogs: χ2 = 36.78, df = 4, P < 0.0001; cats: χ2 = 98.77, df = 4, P < 0.0001). Outdoor domestic dogs and cats exhibited the highest prevalence (cats: 78.5%, 51/65, ASR = 7.3; dogs: 50.0%, 42/84, ASR = 3.4), whereas sheltered individuals had the lowest (cats: 27.5%, 100/364, ASR = - 4.4; dogs: 24.5%, 116/473, ASR = - 5.4). Additionally, dogs in contact with outdoor animals had a significantly higher infestation prevalence compared to those without such contact (χ2 = 7.86, df = 1, P = 0.005; 47.8%, 138/289, ASR = 2.8 vs. 30.6%, 26/85, ASR = − 2.8), and those in contact with cats were more frequently infested (χ2 = 14.282, df = 1, P < 0.0001; 39.6%, 91/230, ASR = 3.8 vs. 24.1%, 70/290, ASR = - 3.8).
In contrast to cats, seasonality significantly influenced flea infestation in dogs (χ2 = 16.49, df = 3, P = 0.001), with the highest prevalence observed in summer (39.1%, 106/271, ASR = 2.4) and autumn (39.5%, 90/228, ASR = 2.3), while the lowest infestation prevalence was in winter (25.1%, 44/175, ASR = − 2.5).
Flea species
A total of 1513 flea specimens were collected from dogs (51.8%, 784/1513) and cats (48.2%, 729/1513) (Fig. 1). Ctenocephalides felis was the most frequently identified flea species, accounting for 85.7% (672/784) of fleas in dogs and 98.8% (720/729) in cats (Table 3). Other species identified included C. canis (7.8%, 61/784 in dogs; 1.2%, 9/729 in cats), P. irritans (5.5%, 43/784, exclusively in dogs) and A. erinacei maura (1.0%, 8/784, exclusively in dogs).
Geographic distribution of flea species detected in infested hosts, by regions classified according to the Nomenclature of Units for Territorial Statistics level II (NUTS II). A Ctenocephalides felis; B Ctenocephalides canis; C Pulex irritans; D Archaeopsylla erinacei maura.
Mono-infestations were predominant, with C. felis being the only flea species in 85.6% (302/353) of infested dogs and 98.2% (372/373) of infested cats. Co-infestations were observed only in dogs (3.4%, 12/353), most frequently involving C. felis and P. irritans (50.0%, 6/12), followed by C. canis and C. felis (33.3%, 4/12), A. erinacei maura and C. felis (8.3%, 1/12) and C. canis, C. felis and P. irritans (8.3%, 1/12).
Flea intensity ranged from 1 to 17 (mean 2.2) fleas per dog and from 1 to 33 (mean 1.9) fleas per cat.
Molecular characterisation and phylogenetics
A total of 75 cox2 sequences were obtained (100% amplification success) from flea specimens morphologically identified as C. felis (72.0%, 54/75), C. canis (13.3%, 10/75), P. irritans (13.3%, 10/75) and A. erinacei maura (1.3%, 1/75) (Figs. 2, 3, 4, 5).
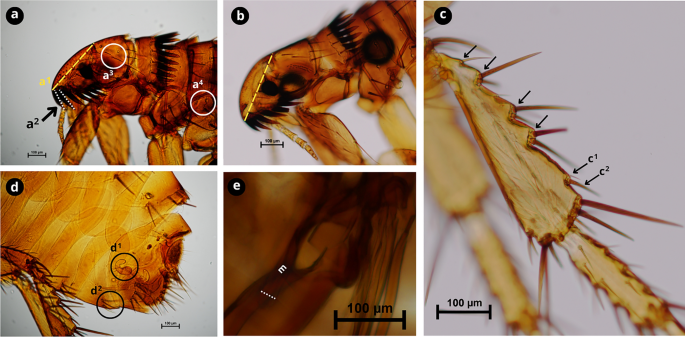
Morphological characteristics of Ctenocephalides felis. a Female cephalic capsule with a noticeable acute angle and not very convex front anteriorly (a1); first spine of the genal comb is approximately the same length as the second (a2); occiput area has two setae (a3); lateral metatorax area with one or two setae (a4). b Male front more convex anteriorly compared to the female. c Hind tibia with five groups of setae bearing notches on the dorsoposterior margin (black arrows) with vestigial spiniform setae c1 and developed seta c2. d Female genitalia; spermatheca (d1); sternite VII with two setae, one posterior and one anterior (d2). e Male genitalia, with the manubrium (m) not expanded apically with a constricted apex (white dashed line). Scale bars as shown in the Figure.
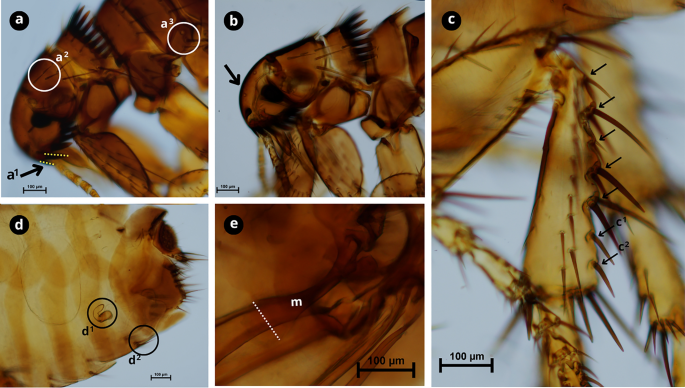
Morphological characteristics of Ctenocephalides canis. a First spine of the genal comb is half the length of the second (a1); occiput area has three setae (a2); lateral metatorax area with three setae (a3). b Cephalic capsule with a convex front anteriorly. c Hind tibia with six to seven groups of setae bearing notches on the dorsoposterior margin (black arrows) with developed spiniform setae c1 and c2. d Female genitalia; spermatheca (d1); sternite VII with two setae on the same level (d2). e Male genitalia, with manubrium (m) expanded apically with a dilated apex (white dashed line). Scale bars as shown in the Figure.
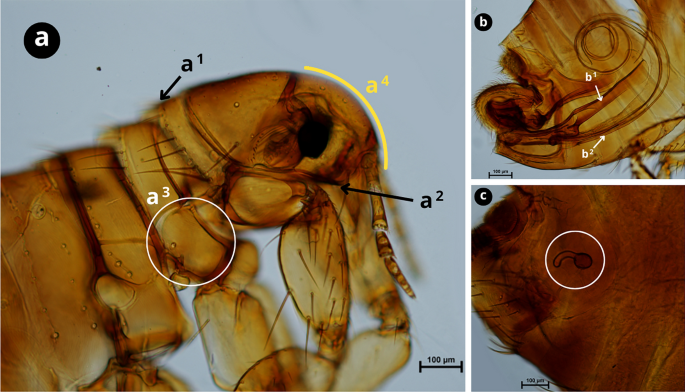
Morphological characteristics of Pulex irritans. a Pronotal and genal combs absent (a1 and a2); pleural rod of mesothorax absent (a3); rounded front (a4). b Male genitalia, phallosome (tubular and central) (b1) and accessory filaments (ventral) (b2). c Female genitalia, spermatheca. Scale bars as shown in the Figure.
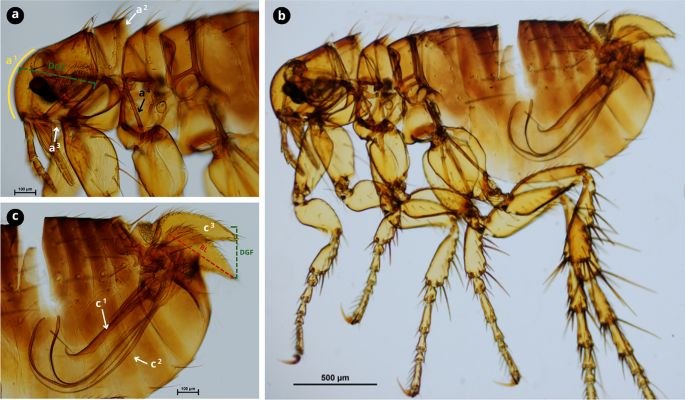
Morphological characteristics Archaeopsylla erinacei maura (male). a Pronotal comb absent or vestigial, with one or three combs on each side (a1); vestigial genal comb with one or three spines (a2); pleural rod of mesothorax present (a3); cephalic capsule with rounded front (a4); distance from base of spine at tip of genal process to front (DGF). b Whole body. c Phallosome (tubular and central) (c1) and accessory filaments (ventral) (c2); basimere (c3); basimere length (higher in the subspecies A. erinacei maura compared to A. erinacei erinacei) (BL), basimere length equal to DGF. Scale bars as shown in the Figure.
Phylogenetic analysis indicated that C. felis sequences shared a common ancestor. Nonetheless, C. felis felis showed a paraphyletic origin, with sequences obtained from morphologically identified C. felis specimens collected from dogs and cats segregating into a monophyletic cluster with low genetic divergence, composed exclusively of reference sequences of C. felis felis from dogs and cats in Australia, Hungary, Israel, Italy and Spain (Fig. 6).
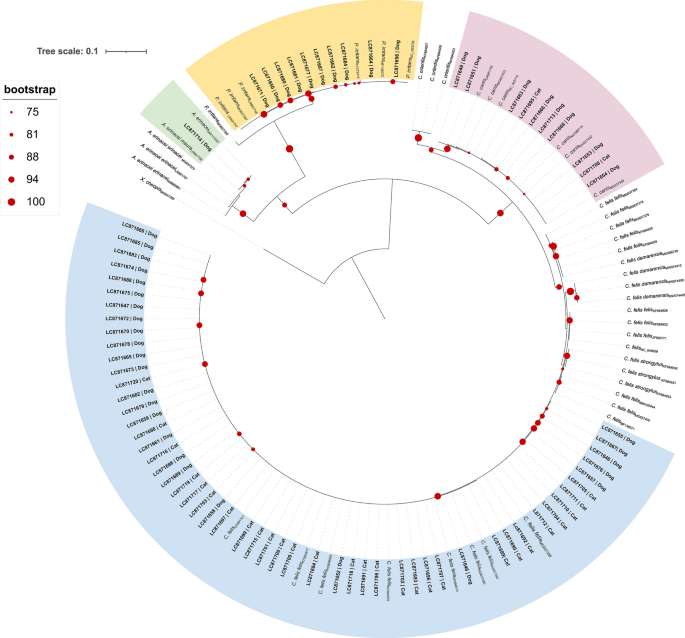
Maximum likelihood phylogenetic tree inferred from cytochrome oxidase subunit II (cox2) sequences of various flea species and subspecies. Tree reconstruction was performed in IQ-TREE using the K3Pu+F+G4 substitution model, selected as the best-fitting model based on the Bayesian Information Criterion. Node support was assessed using 1000 bootstrap replicates, and values ≥75% are shown at the corresponding nodes. The tree was rooted using Xenopsylla cheopis sequence (outgroup). Reference sequences are labelled with species name and GenBank accession number. Sequences obtained in this study are shown in bold and include specimen identifier and GenBank accession number (LC871646-LC871720). Branch lengths are scaled to the number of substitutions per site. Coloured sectors denote the phylogenetic clusters in which the sequences obtained in this study segregated
Ctenocephalides canis showed a monophyletic origin, with the obtained sequences of C. canis from dogs and cats clustering together with reference sequences of C. canis collected from dogs and cats in China, the Czech Republic, Hungary, Iran and Turkey.
The obtained sequences of P. irritans from dogs segregated into a monophyletic cluster composed exclusively of sequences of P. irritans collected from humans and animals, Argentina, China, Croatia, Madagascar and Spain.
Similarly, the obtained sequence of A. erinacei maura collected from a dog formed a robust monophyletic cluster with sequences of A. erinacei maura from hedgehogs in Spain and Portugal.
Flea allergy dermatitis in infested animals
Flea allergy dermatitis was significantly associated with flea infestation in both dogs and cats (dogs: χ2 = 71.81, df = 1, P < 0.0001; cats: χ2 = 16.02, df = 1, P < 0.0001. Infested animals had a higher prevalence of FAD compared to non-infested individuals (dogs: 16.1%, 53/330, ASR = 8.5 vs. 1.8%, 12/653, ASR = − 8.5); cats: 5.4%, 20/367, ASR = 4.0 vs. 1.1%, 7/616, ASR = - 4.0). Host species was also significantly associated with FAD occurrence (χ2 = 16.47, df = 1, P < 0.0001), with dogs showing a higher prevalence of FAD (6.6%, 65/983, ASR = 4.1) compared to cats (2.7%, 27/983; ASR = − 4.1). In addition, flea burden was significantly higher in animals presenting clinical signs compatible with FAD (dogs: U = 15098.00, Z = − 7.95, P < 0.0001; cats: U = 8168.00, Z = − 3.79, P < 0.0001).
Effect of insecticide use
The use of insecticides was significantly associated with lower flea infestation in both dogs and cats (dogs: χ2 = 23.69, df = 1, P = < 0.001; cats: χ2 = 21.78, df = 1, P = < 0.001).
Infestation prevalence was lower in treated animals (dogs: 22.4%, 146/651, ASR = − 4.9; cats: 21.7%, 106/488, ASR = = − 4.7) compared to untreated individuals (dogs: 50.0%, 32/64, ASR = 4.9; cats: 45.8%, 38/83, ASR = = 4.7), indicating a protective effect of insecticide application. Additionally, a significant association was observed between insecticidal use and flea infestation in both species (P < 0.001). Animals treated with fipronil showed the highest ASR (dogs = 6.0; cats = 8.7), compared to all other treatment groups (Additional file 2: Table S1, Table S2).
Multivariable logistic regression analysis of risk factors for flea infestation
The multivariate logistic regression analysis identified NUTS II/seasonality/insecticide use and NUTS II/lifestyle/insecticide use as significant predictors of flea infestation in dogs (G2 = 85.22, df = 8, P < 0.0001) and cats (G2 = 136.12, df = 9, P < 0.0001), respectively (Additional file 2: Table S3, Table S4; Figs. 7, 8).
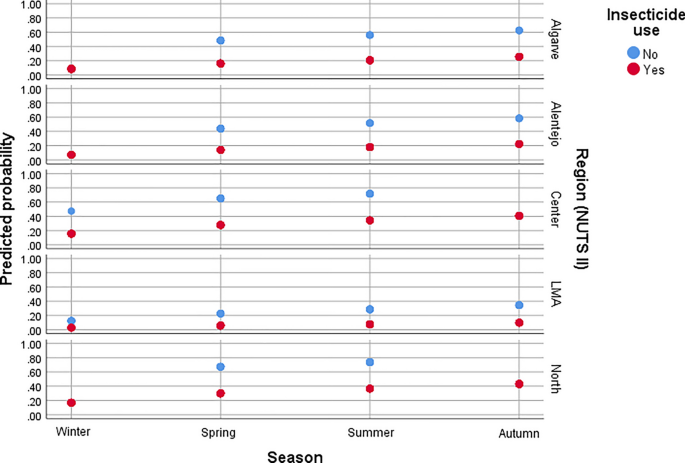
Predicted probability of flea infestation in dogs according to NUTS II region, season, and insecticide use.
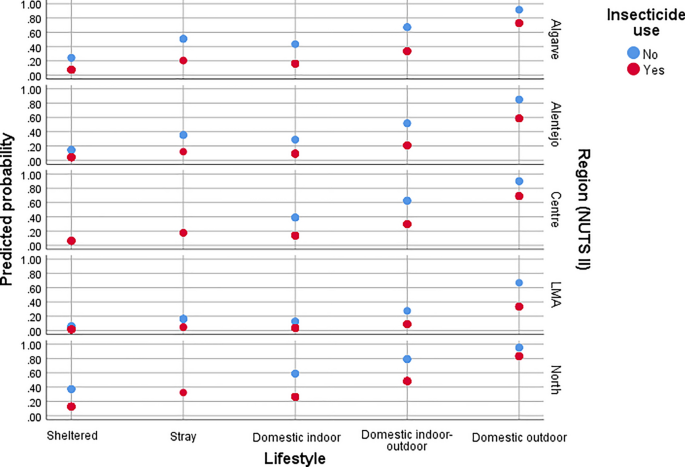
Predicted probability of flea infestation in cats according to NUTS II region, lifestyle, and insecticide use.
Regarding infestation according to NUTS II regions in mainland Portugal, compared to the North region, the odds of flea infestation were significantly lower in LMA (χ2Wald = 31.85, df = 1, P < 0.0001, aOR = 0.14, 95% CI 0.21–0.69), Alentejo (χ2Wald = 10.35, df = 1, P = 0.001, aOR = 0.38, 95% CI 0.21–0.69) and Algarve (χ2Wald = 8.81, df = 1, P = 0.003, aOR = 0.45, 95% CI 0.27–0.76) for dogs, and in LMA (χ2Wald = 24.73, df = 1, P < 0.0001, aOR = 0.10, 95% CI 0.41–0.25), Alentejo (χ2Wald = 10.08, df = 1, P = 0.001, aOR = 0.28, 95% CI 0.13–0.62), and Centro (χ2Wald = 7.08, df = 1, P = 0.008, aOR = 0.45, 95% CI 0.25–0.81) for cats.
The odds of flea infestation in dogs were significantly higher in spring (χ2Wald = 4.60, df = 1, P = 0.032, aOR = 2.08, 95% CI 1.07–4.06), summer (χ2Wald = 8.89, df = 1, P = 0.003, aOR = 2.83, 95% CI 1.43–5.61) and autumn (χ2Wald = 13.85, df = 1, P < 0.0001, aOR = 3.72, 95% CI 1.86–7.43), compared to winter.
In cats, lifestyle was a significant predictor of flea infestation (χ2Wald = 53.93, df = 4, P < 0.0001), with domestic cats having outdoor access exhibiting the highest odds of infestation.
Flea infestation was associated with the absence of insecticide use in both dogs and cats. Compared to treated individuals, untreated dogs had nearly a fivefold higher odds of infestation (χ2Wald = 27.57, df = 1, P < 0.0001, aOR = 4.87, 95% CI 2.70–8.79), while untreated cats had approximately a fourfold higher odds of infestation (χ2Wald = 17.88, df = 1, P < 0.0001, aOR = 4.02, 95% CI 2.11–8.67).
The adjusted models demonstrated a good fit to the data (dogs: χ2HL = 7.96, df = 8, P = 0.438; cats: χ2HL = 9.72, df = 7, P = 0.205) and exhibited adequate discriminative performance (dogs: AUC = 0.71, P < 0.0001; cats: AUC = 0.77, P < 0.0001).