This review provides an in-depth analysis of the epidemiology and geographical distribution of NTM species in Ethiopia, highlighting their diversity, geographical variations, and clinical relevance. It is the first of its kind in the region, indicating that NTM species distribution significantly varies by geography, with significant clinical implications [29, 30].
Hoefsloot et al.’ 2008 worldwide collection of NTM isolates includes solely samples from South Africa, a sub-Saharan African country [31]. Kendall et al.‘s second update in 2013 did not considerably increase the inclusion of African NTM isolates [16]. Furthermore, Catherine et al. 2017 did a comprehensive review and meta-analysis of NTM isolated from sputum samples in Sub-Saharan Africa, which included only three studies from Ethiopia [32]. This demonstrates a continuing gap in Ethiopia’s representation in NTM data, emphasizing the need for regional research initiatives to better understand the epidemiology of these illnesses.
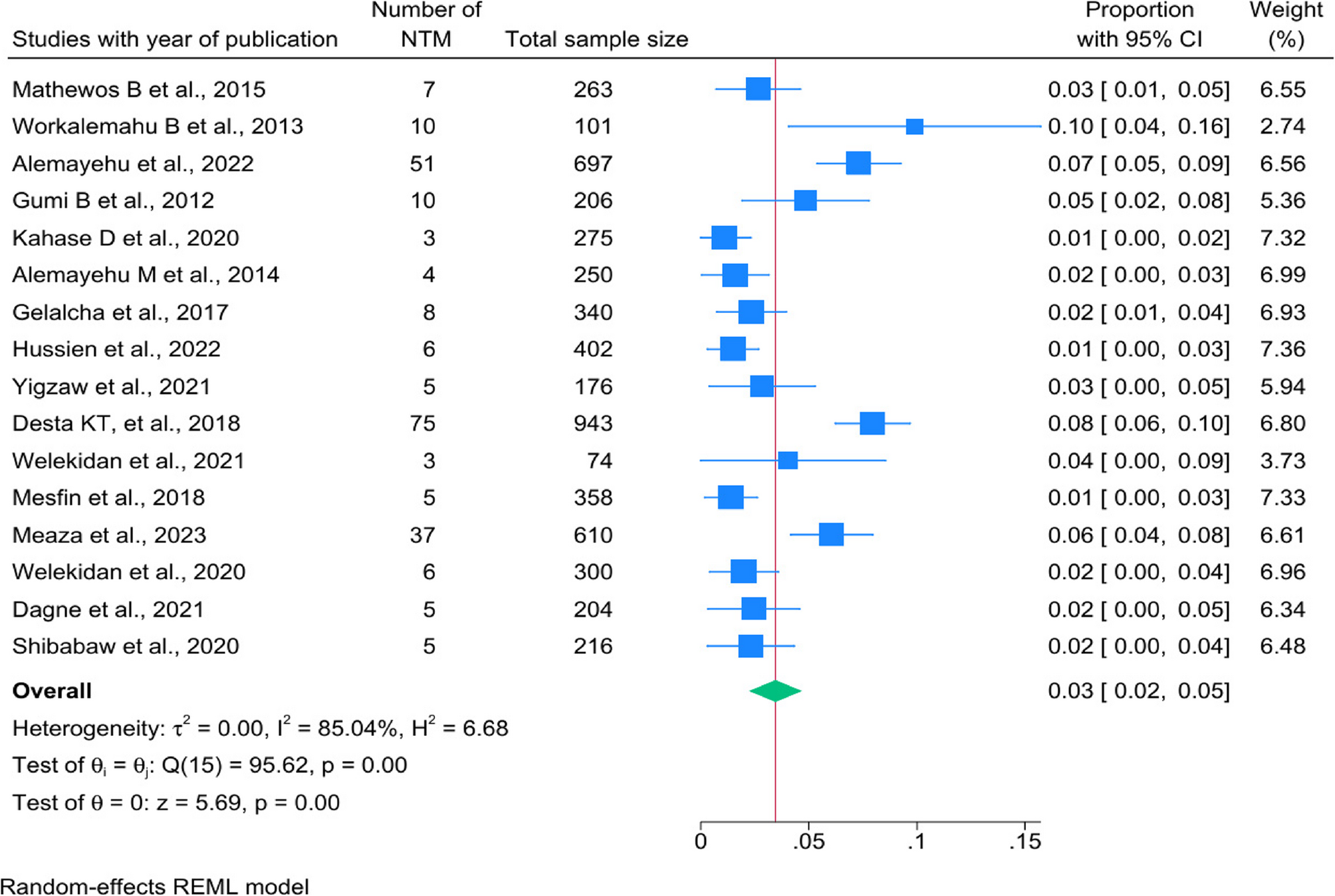
The estimated prevalence of NTM in Ethiopia was 3.8% (95% CI: 2.5–5.1%, I² =85.04%, p < 0.001). These findings fall within the range specified in African literature reviews, which indicate that the prevalence of NTM in Africa ranges from 0.2–28% [14]. However, this prevalence is lower than the global prevalence of NTM, which stands at 10% [33] and Sub-Saharan Africa among HIV patients 7.5% [34]. It is also lower than reports from other African regions: Ghana at 23% [35], Nigeria ranging from 1–36% [34, 35], Uganda at 9.2% [34], Tanzania at 15.0% [34], and Zambia ranging from 15.8–25.8% [32]. The lower frequency of NTM in Ethiopia is likely due to environmental factors, diagnostic limitations, epidemiological differences, and research design limitations. Enhanced diagnostics, targeted surveillance in high-risk groups, and environmental studies are needed to clarify NTM epidemiology. In other case, NTM infections are often misdiagnosed as tuberculosis due to their similar clinical presentations, which can result in inappropriate treatment [14].
In our study, the prevalence of NTM infection in Addis Ababa varied from 1 to 8%, with a pooled estimate of 4%. Similarly, Oromia exhibited a range from 1 to 10%, also with a pooled estimate of 4% [2, 27, 28, 36, 37]. The cumulative proportion with NTM in our study was higher than the study done in, Mali 3.4% [38], in Pakistan 0.7% [39] and in India 1.2% [40], yet lower than the 6.4% observed in China [41]. The observed prevalence in these regions can be attributed to several factors, including the higher population density in these areas, which increases the likelihood of detecting and reporting NTM cases. Furthermore, Addis Ababa and Oromia are recognized to having more advanced investigative capabilities, which may lead to increased identification rates of NTM infections. Also, this result might be attributable to geographical variations in the prevalence of these mycobacteria, as well as the diverse spectrum of infections they cause across different regions worldwide [31, 32].
This study performed a subgroup analysis of NTM across different age categories, including children, adults, and all age groups together. The analysis revealed that the overall pooled prevalence of NTM across all age groups was 5% (95% CI: 0.02–0.07, I² = 91.10%), which is higher than the prevalence reported in other specific age categories [2, 28, 42,43,44,45]. In contrast, a single study focusing on children reported a prevalence of 10% (95% CI: 0.04–0.16) [26]. This contrasts with findings from studies conducted in Mali, where a greater number of cases were reported among older individuals [38]. Also, these are lower than those reported in some Sub-Saharan African studies like Zambia found a prevalence of 14.8% among cattle-exposed children—likely linked to soil and water contamination [46]—while our findings are higher than the 1–3% prevalence typically reported in high-income countries such as those in Europe and the USA [33]. This may be attributable to the larger number of participants in the combined group, discrepancies in diagnostic systems leading to inconsistencies, and host as well as environmental factors—mostly, the increased exposure of children due to greater soil and water contact [46].
The subgroup study’s finding of a pooled 4% (95% CI: 0.02–0.06) NTM detection rate using culture methods, with high heterogeneity (I² = 86.71%) [28, 37, 42, 44, 47, 48], reveals significantly lower when compared to regional and global data, particularly from SSA 7.5% colonization prevalence [32], Ghana 8% among HIV-positive adults [32]. These variations might be due to methodological, epidemiological, and environmental influences on NTM detection.
This high heterogeneity indicates considerable variability among the study-specific proportions, which ranged from 1 to 8%. Similarly, molecular methods showed a pooled proportion of 4% (95% CI: 0.02–0.06) accompanied by very high heterogeneity (I² = 88.33%) [2, 25, 27, 47,48,49,50]. The variability in proportions for molecular methods was even more pronounced, ranging from 1 to 10%. The observed difference in both culture and molecular methods may be due to differences in diagnostic accuracy (sensitivity, specificity, positive predictive value, and negative predictive value), regional variances, publication bias, variations in patient populations, and differing diagnostic criteria affecting detection rates.
This review analyzing NTM prevalence in PTB patients showed significant heterogeneity across studies. In this subgroup analysis, the pooled prevalence of NTM among PTB patients was determined to be 3% [2, 37, 43, 47, 50, 51]. This estimate aligns with prevalence figures from high-TB-burden regions—for example, 3.2% in West/Central Africa [35]. However, the prevalence in our analysis exceeds those reported from Nigeria (1%) [35] and Cameroon (1.3%) [35], while it remains lower than the 8.4% reported in Ghana [32]. This variation may be attributable to a combination of demographic and environmental influences, as well as methodological disparities. Especially, the application of molecular diagnostic techniques has markedly increased detection rates; like molecular methods have reported a positivity rate of 25.2% [35], in contrast to the 8.4% [32] positivity observed with traditional culture-based methods.
The review also showed that NTM prevalence in PTB suspected patients was 0.04, with a high heterogeneity of 85.98% [25,26,27,28, 36, 42, 44, 48]. This substantial variation suggests that differences in study designs, patient populations, and diagnostic methods might have influenced the results. Also, factors such as differences in the clinical criteria for suspecting TB, the diagnostic tools used, and the study settings could all play a role in the observed variability.
In Addis Ababa, the predominant NTM species isolated in this review was M. fortuitum, M. simiae, M. abscessus, M. intracellulare, and M. peregrinum. Among these, M. fortuitum and M. abscessus are recognized for their rapid growth. Similarly, studies have found a predominance of Mycobacterium avium complex (MAC) in the epidemiology of NTM in parts of Australia and North America [16] and Kenya and other Africa country [34]. Also, colder regions like northern Japan exhibit majorly M. abscessus dominance [52], while due to soil factors with high sodium/manganese containing countries like U.S. and Japan reported in similar rate [46]. On the other hand, Oromia is predominantly occupied by M. fortuitum, M. parascrofulaceum, M. triviale, and M. intracellulare. These findings align with studies conducted in sub-Saharan Africa [32]. This might be due to diagnostic methods, co-morbidity and environmental factors [32, 35].
Many rarely isolated NTM species, such as M. triviale, M. simiae, M. flavescens, M. elephantis, M. gordonae, and M. peregrinum, have also been identified in presumptive tuberculosis patients. While some of these species have been isolated in this study with chronic bronchitis, pulmonary tuberculosis, sub-acute pneumonia, and healed tuberculosis in other parts of the world, it remains unclear what role they play in the etiology of pulmonary disease in Ethiopia [36].
The findings of this study underline critical gaps in Ethiopia’s diagnostic capacity for non-tuberculous mycobacteria (NTM), necessitating policy-driven interventions to refine detection strategies, standardize diagnostic frameworks, and optimize laboratory workflows [32]. Given the high reliance on culture-based methods, the current diagnostic infrastructure misses a substantial proportion of NTM cases—culture-based detection pooled at 4% (I² = 86.71%) [28, 37, 42, 44, 47, 48], while molecular assays in comparable settings (e.g., Ghana) yield 25.2% positivity versus 8.4% by culture [32]. Scaling up molecular diagnostics, including PCR, sequencing, and line probe assays (e.g., GenoType CM/AS), is crucial to improve accuracy and address the isolates remaining unidentifiable due to methodological constraints [14]. Hence, future studies should standardize NTM identification by applying universal guidelines with rigorous specimen collection protocols and combining culture methods with advanced molecular techniques, such as PCR and gene sequencing. Establishing centralized reference laboratories, quality assurance programs, and harmonized data reporting will ensure reproducibility, comparability, and ultimately robust epidemiological insights [53, 54].
Limitations
The study has limitations due to high heterogeneity in studies in their designs, patient populations, sampling strategies, and laboratory methodologies limiting direct comparability. Furthermore, the generalizability of identified patterns across Ethiopia is limited because certain regions lacked any data at all. Also, there is a potential publication bias suggesting that negative or non-significant findings are still underestimated, which could distort the perceived prevalence and distribution of NTM species. Furthermore, diagnostic challenges, including over-reliance on smear microscopy, slow culture methods, delaying diagnosis, limited access to molecular techniques, inconsistent application of diagnostic criteria, and systemic gaps in laboratory capacity and quality assurance limited the species identification and prevalence estimates. Additional constraints include, the predominance of cross-sectional studies, with a complete lack of longitudinal data, hindering understanding of infection dynamics, treatment outcomes, and true clinical significance; inconsistent and incomplete reporting of comorbidities (especially HIV status and underlying lung disease), limiting the ability to assess key risk factors and clinical associations; and small sample sizes in many studies reduce confidence in the pooled estimates of NTM prevalence and distribution.