This cross-sectional study reports the prevalence and genotype distribution of human papillomavirus infection among women in Yangpu District, Shanghai, China from 2020 to 2024. The overall HPV infection rate detected in this study was 23.10%. Compared with similar studies in China, this infection rate is higher than the 21.0% reported in Beijing [11], 21.97% in Jinshan District Shanghai [12], 22.82% in Luoyang Henan Province [13], and 17.92% in Zhoupu District Shanghai [14], but lower than the 41.04% in Hangzhou Zhejiang Province and 50.64% in Tianjin [15, 16]. This discrepancy may be associated with variations in regional epidemiological characteristics or demographic composition of study populations [17]. Previous studies have reported substantial heterogeneity in HPV prevalence across China, ranging from 6.2–50.64% [16, 18]. Prevalence rates in clinical populations significantly exceed those in community-based screening cohorts, likely reflecting health-seeking behaviors related to symptomatic presentation that differ fundamentally from asymptomatic screening populations [19]. In the present study, an HPV prevalence of 10.5% was observed among general screening populations, lower than the 13.6% rate reported in routine screening cohorts from Zhejiang Province [20].
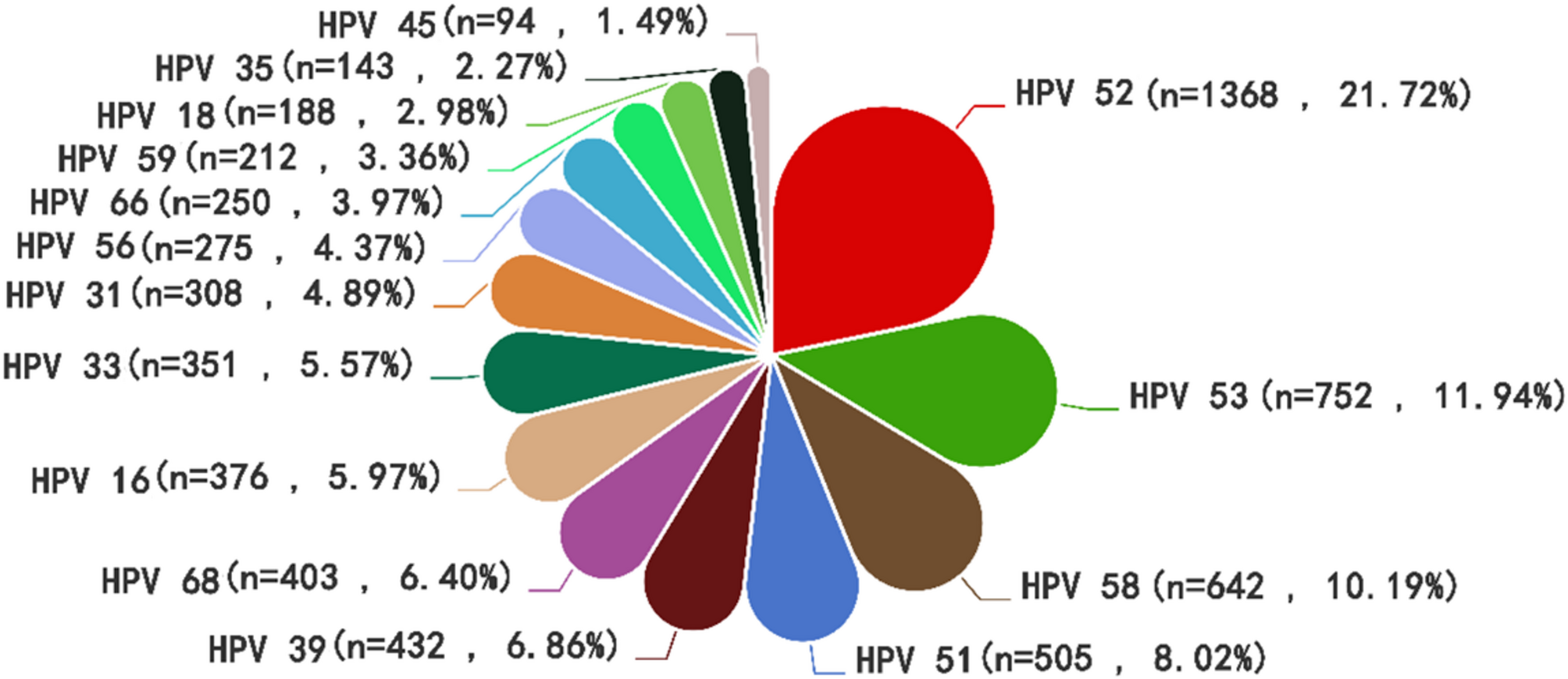
This regional analysis identified HPV-52 as the predominant high-risk genotype, followed by HPV-53, -58, -51, -39, and − 68, aligning with patterns reported in other Chinese studies. HPV-52, -16, -58, -51, and − 66 represent predominant subtypes in Beijing [11], while HPV-52, -16, -58, -51, and − 53 dominate in Jinshan District, Shanghai [12]. Similarly, HPV-52, -58, -16, -53, and − 51 constitute major subtypes in the Golden Triangle region of Fujian, China [3]. Substantial evidence confirms that beyond HPV-16 and − 18, genotypes including HPV-52, -58, -51, and − 53 significantly contribute to cervical carcinogenesis [21]. This study’s findings exhibit discrepancies in subtype prevalence rankings compared with certain Chinese regions [22], indicating significant geographical heterogeneity in high-risk HPV genotype distributions. This epidemiological variation likely originates from multifactorial mechanisms: Firstly, socio-behavioral patterns—including number of sexual partners, marital/reproductive history, and sexual health literacy—fundamentally shape subtype distributions by modulating viral transmission routes and exposure frequency. Secondly, molecular interactions between HPV genotypes and region-specific host immunogenetic backgrounds may establish distinct infection profiles in defined populations [23].
From the perspective of infection patterns, HPV infections exhibit a phenomenon of multi-subtype coexistence. Beyond single-subtype infections, mixed-infection modes are particularly prominent, with dual infections being the predominant form. It remains unclear whether co-infection with multiple HPV genotypes involves competitive or synergistic relationships. However, existing studies indicate that mixed infections with multiple types increase the risk of cervical cancer more significantly than single-genotype infections [24, 25]. A study conducted in Mexico observed a correlation between multiple HPV infections and high viral load as well as infection persistence [26]. Research from South Korea demonstrated that patients with multiple HPV infections had longer viral clearance cycles compared to those with single-type infections [27]. The mechanisms and potential carcinogenic effects of multiple infecting genotypes require further investigation. Fig. 2 results revealed that HPV-52, HPV-53, and HPV-58 were the most common genotypes in co-infections among women in this study, with HPV-52 + HPV-53 and HPV-52 + HPV-58 co-infections being the most frequent combinations. In Shanghai, China, HPV vaccination began in 2017, and Yangpu District started the program in 2018. Initially, the bivalent vaccine targeting HPV-16 and − 18 and the quadrivalent vaccine covering HPV − 6, -11, -16, and − 18 were mainly provided. In October of the same year, the nine-valent HPV vaccine, which covers more high-risk types including HPV − 6, -11, -16, -18, -31, -33, -45, -52, and − 58, was officially made available for vaccination. Vaccination mainly relies on the principle of self-payment and voluntary participation, and the overall vaccination rate remains relatively low. Among the HPV vaccines available on the market, only the nine-valent vaccine covers HPV-52 and HPV-58, making it more suitable for women in Yangpu District, Shanghai.
Given that the HPV-53 vaccine is still in the preclinical research stage, its high prevalence in certain regions further highlights the insufficiency of the existing vaccine’s coverage. Currently available vaccines are all based on HPV L1 self-assembled virus-like particles (VLPs), which can prevent HPV infection by inducing the production of specific neutralizing antibodies in the body [28]. Although these vaccines have shown significant efficacy, they still have several limitations, including a limited coverage of HPV types, the need to produce VLPs of different types separately before mixing them, and a high dependence on cold chain transportation and storage. The high prevalence of HPV-53 indicates the insufficiency of the current vaccine strategy in terms of protection spectrum. Therefore, in the future, it is urgent to expand the vaccine coverage through technological innovation, such as developing cross-protective vaccines based on L2 protein or constructing new multivalent vaccine systems using DNA and mRNA platforms. Although these emerging technologies face challenges in terms of research and development difficulty and cost control, they have significant potential in improving the broad-spectrum and accessibility of vaccines. The development of vaccines targeting HPV-53 is expected to provide more targeted prevention measures for regions with high prevalence.
Multiple studies have confirmed that HPV infection rates in China exhibit a U-shaped curve distribution with age, characterized by two distinct peaks [11, 24]. In this study, the first peak occurred in the under-20 age group, which may be associated with evolving sexual attitudes among youth in the current social context. A large-scale cross-sectional survey of Chinese women aged 15–24 revealed that the median age of first sexual intercourse in this cohort was 17 years [29]. Another epidemiological study demonstrated that within two to three years after initial sexual activity, HPV infection rates among adolescents can reach 50–80%, with a corresponding 2.41-fold increase in infection risk [30]. Although the sample size of the under-20 population in this study was limited, their significant disease risk profile indicates the necessity of HPV screening for this age group. The second peak emerged in the 61–70 age group, likely attributed to age-related declines in immune function and hormonal changes, which increase HPV susceptibility and reduce viral clearance capacity [31, 32]. The differential infection patterns across age groups underscore age as a critical influencing factor for HPV infection, necessitating age-specific screening strategies. Particularly in the 61–70 cohort, the marked elevation in HPV infection rates suggests these individuals should be prioritized as key surveillance targets..
Figure 3 results show that HPV-52, HPV-53, and HPV-58 were the predominant subtypes, with their infection rates maintaining high levels across all age groups. The peak infection rates occurred in the ≤ 20 years and 61–70 years age groups, but positive infections for various HPV types were primarily concentrated in the 31–40 years, 51–60 years, and 61–70 years age groups. This indicates that the population actively undergoing gynecological examinations is mainly composed of middle-aged and elderly women, which is associated with increased awareness of HPV infection among this demographic due to the introduction and promotion of HPV vaccines in recent years [22]. From a prevention and control strategy perspective, HPV vaccination and screening are core measures to reduce cervical cancer risk. Data demonstrate that the earlier young women receive the HPV vaccine, the higher the antibody titer and the better the protective effect of the vaccine [33]. However, HPV vaccination coverage in China remains critically low at approximately 2.64–11.0%, significantly below immunization rates observed in most other countries [8, 34, 35]. Multiple obstacles have led to this low vaccination rate. Among female college students, insufficient awareness of the risk of HPV infection and concerns about the safety and efficacy of the vaccine are the main obstacles [36]. Concurrently, current vaccine shortages and exclusion from the national immunization program likely impede or delay individual vaccination decisions [37]. Consequently, stratified immunization and screening strategies informed by age-specific prevalence patterns should be implemented to address differential HPV exposure across age cohorts.
This study constitutes a cross-sectional analysis based on HPV testing data from 2020 to 2024 at Shanghai Yangpu District Shidong Hospital, systematically elucidating the epidemiological characteristics of HPV in Yangpu area. It supplements the multi-center epidemiological database of Shanghai and provides scientific evidence for regional prevention policies. However, the following limitations exist: Firstly, the study cohort solely comprised hospital patients within this five-year timeframe, which is not representative of the typical local population. Secondly, HPV vaccination history was not incorporated into the dataset, whereas existing evidence indicates that vaccination status significantly influences the infection spectrum distribution of high-risk HPV subtypes. Finally, the absence of synchronized collection of cervical cytology or histopathological diagnosis results restricted the analysis of associations between HPV genotype distribution patterns and cervical lesion severity. As a retrospective single-center study, our research sample included only patients seeking hospital care. This design excludes potentially infected individuals within the community who did not present for medical attention. This may limit the generalizability of our findings to the broader community population. Therefore, caution is warranted when extrapolating these conclusions to wider populations. Future investigations should expand sample size, enhance data dimensionality, and adopt multi-center collaborative models to systematically explore the association mechanisms between HPV infection and cervical lesions in Yangpu area..