image:
Researchers from Shibaura Institute of Technology have designed and synthesized novel vitamin K analogues conjugated with retinoic acid, which exhibit potent neuronal differentiation-inducing activities. Their findings highlight unique mechanisms underlying the neuroprotective effects of vitamin K and its analogues, paving the way for the development of effective treatments against neurodegenerative diseases.
view more
Credit: Associate Professor Yoshihisa Hirota, Shibaura Institute of Technology, Japan
Source Link: https://pubs.acs.org/doi/full/10.1021/acschemneuro.5c00111
Neurodegenerative diseases like Alzheimer’s, Parkinson’s, and Huntington’s disease are characterized by the progressive loss of neurons. The resulting debilitating symptoms, such as loss of memory and cognition, and motor impairment, can significantly degrade patients’ quality of life, confining them to round-the-clock care. While currently used drugs help alleviate symptoms, curative treatments are lacking, thus underscoring the need for novel therapeutic strategies. One such strategy involves the induction of neuronal differentiation, which can replenish lost neurons and potentially stall or reverse neurodegeneration.
Vitamin K, a fat-soluble vitamin with established roles in blood coagulation and bone metabolism, has been recently implicated in neuronal differentiation and neuroprotection. However, the therapeutic activity of naturally active vitamin K compounds like menaquinone 4 (MK-4) may be insufficient for their application in regenerative medicine against neurodegenerative diseases.
In a new pioneering study published online in the journal ACS Chemical Neuroscience on July 03, 2025, a team of researchers led by Associate Professor Yoshihisa Hirota and Professor Yoshitomo Suhara from the Department of Bioscience and Engineering, Shibaura Institute of Technology, Japan, has designed and synthesized novel vitamin K analogues with enhanced neuroactive properties. Also, they report a unique mechanism of action by which vitamin K induces neuronal differentiation.
Giving further insight into their work, Dr. Hirota explains, “The newly synthesized vitamin K analogues demonstrated approximately threefold greater potency in inducing the differentiation of neural progenitor cells into neurons compared to natural vitamin K. Since neuronal loss is a hallmark of neurodegenerative diseases such as Alzheimer’s disease, these analogues may serve as regenerative agents that help replenish lost neurons and restore brain function.”
To improve the potency of vitamin K, the researchers synthesized 12 vitamin K hybrid homologs conjugated with retinoic acid—an active metabolite of vitamin A known to promote neuronal differentiation, a carboxylic acid moiety, or a methyl ester side chain and compared the neuronal differentiation-inducing activity of the hybrid homologs.
Vitamin K and retinoic acid regulate transcriptional activity via the steroid and xenobiotic receptor (SXR) and retinoic acid receptor (RAR), respectively. The researchers assessed SXR and RAR transcriptional activity in mouse neural progenitor cells treated with the newly synthesized compounds. Notably, the biological activity of vitamin K and retinoic acid was preserved in the hybrid homologs. Further, the researchers examined the differentiation of neural stem cells treated with the homologs by quantifying the expression of microtubule-associated protein 2 (Map2), a marker of neural growth expressed by neurons. Compound that possessed both the conjugated structure of retinoic acid and a methyl ester side chain exhibited a three-fold higher neuronal differentiation activity compared with the control and significantly higher activity than natural vitamin K compounds, hereafter referred to as Novel vitamin K analog (Novel VK).
To further elucidate the mechanism by which vitamin K exerts neuroprotective effects, the researchers compared the gene expression profiles of neural stem cells treated with MK-4, a neuronal differentiation-inducing compound, and a compound that suppresses the differentiation of stem cells into neurons. The transcriptomic analysis revealed that metabotropic glutamate receptors (mGluRs) mediate vitamin K-induced neuronal differentiation through downstream epigenetic and transcriptional regulation. The effects of MK-4 were specifically mediated by mGluR1. Notably, mGluR1 has been previously implicated in synaptic transmission, and mGluR1-deficient mice exhibit motor and synaptic dysfunction, which are characteristic features of neurodegenerative diseases.
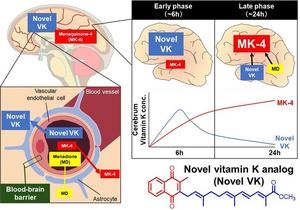
Delving deeper, the researchers conducted structural simulations and molecular docking studies to elucidate whether the vitamin K homolog interacts with mGluR1. Indeed, their analysis revealed a stronger binding affinity between Novel VK and mGluR1. Finally, the researchers examined the cellular uptake of Novel VK and its conversion to bioactive MK-4 in cells and mice. They noted a significant concentration-dependent increase in the intracellular concentration of MK-4. Moreover, Novel VK converted to MK-4 more easily than natural vitamin K. Further, in vivo experiments in mice showed that Novel VK exhibited a stable pharmacokinetic profile, crossed the blood-brain barrier, and achieved higher MK-4 concentration in the brain compared to the control.
Overall, the study sheds light on the mechanism by which vitamin K and its structural analogues exert neuroprotective effects, paving the way for the development of novel therapeutic agents that can delay or reverse neurodegenerative diseases.
Concluding with the long-term implications of their work, Dr. Hirota says, “Our research offers a potentially groundbreaking approach to treating neurodegenerative diseases. A vitamin K-derived drug that slows the progression of Alzheimer’s disease or improves its symptoms could not only improve the quality of life for patients and their families but also significantly reduce the growing societal burden of healthcare expenditures and long-term caregiving.”
We hope their research translates into clinically meaningful treatments for patients battling neurological diseases.
***
Reference
DOI: 10.1021/acschemneuro.5c00111
About Shibaura Institute of Technology (SIT), Japan
Shibaura Institute of Technology (SIT) is a private university with campuses in Tokyo and Saitama. Since the establishment of its predecessor, Tokyo Higher School of Industry and Commerce, in 1927, it has maintained “learning through practice” as its philosophy in the education of engineers. SIT was the only private science and engineering university selected for the Top Global University Project sponsored by the Ministry of Education, Culture, Sports, Science and Technology and had received support from the ministry for 10 years starting from the 2014 academic year. Its motto, “Nurturing engineers who learn from society and contribute to society,” reflects its mission of fostering scientists and engineers who can contribute to the sustainable growth of the world by exposing their over 9,500 students to culturally diverse environments, where they learn to cope, collaborate, and relate with fellow students from around the world.
Website: https://www.shibaura-it.ac.jp/en/
About Associate Professor Yoshihisa Hirota from SIT, Japan
Dr. Yoshihisa Hirota is an Associate Professor at the Shibaura Institute of Technology, affiliated with the Department of Bioscience and Engineering, College of Systems Engineering and Science. He has conducted international research as a Visiting Scholar at the University of Cincinnati. Dr. Hirota’s research focuses on Medicinal Science and Nutritional Biochemistry, with a particular emphasis on the roles of fat-soluble vitamins and nucleic acids in biological systems. With 56 publications to his name, Dr. Hirota’s expertise bridges molecular biology and nutrition, with the goal of extending the healthy life expectancy of the world and leading to better healthcare solutions.
About Professor Yoshitomo Suhara from SIT, Japan
Dr. Yoshitomo Suhara is a Professor at the Shibaura Institute of Technology, affiliated with the Department of Bioscience and Engineering, College of Systems Engineering and Science. His research focuses on medicinal chemistry and drug discovery, with a particular emphasis on the development of bioactive small molecules derived from fat-soluble vitamins, especially vitamins D and K. With over 100 peer-reviewed publications and several patent applications, Dr. Suhara leads multidisciplinary projects involving the design of neurogenic compounds to promote neuronal differentiation, the development of antiviral agents, and the creation of novel anti-cancer molecules.
Funding information
This study was partly supported by a fund for the Mishima Kaiun Memorial Foundation and the Suzuken Memorial Foundation, KOSÉ Cosmetology Research Foundation, Koyanagi Foundation, Research Grants from the Toyo Institute of Food Technology, the Science Research Promotion Fund and the Takahashi Industrial and Economic Research Foundation. This study was partly supported by a Fund for the Promotion of Joint International Research (Fostering Joint International Research (A)) [grant number 18KK0455] and a Grant-in-Aid for Scientific Research (C) [grant numbers 20K05754 and 18K11056, 21K11709, and 24K14656], Grant-in-Aid for Early-Career Scientists [grant number 23K14091] from the Japan Society for the Promotion of Science (JSPS).
Journal
ACS Chemical Neuroscience
Method of Research
Experimental study
Subject of Research
Animals
Article Title
A New Class of Vitamin K Analogues Containing the Side Chain of Retinoic Acid 1 Have Enhanced Activity for Inducing Neuronal Differentiation
Article Publication Date
3-Jul-2025
COI Statement
The authors declare no competing financial interests.