A new review suggests CoQ10 could rejuvenate aging oocytes and enhance embryo quality, offering hope for women facing age-related fertility challenges while spotlighting the need for rigorous clinical trials.
Review: Exploring the protective effects of coenzyme Q10 on female fertility. Image Credit: Adisak Riwkratok / Shutterstock
In a recent review in the journal Frontiers in Cell and Developmental Biology, researchers collated and synthesized the literature on coenzyme Q10 (CoQ10), a crucial antioxidant and energy-producing molecule, and its role in female fertility.
The review leveraged the proceeds of almost 80 previous publications and found that CoQ10 supplementation can enhance ovarian function and improve egg and embryo quality by boosting mitochondrial health and combating oxidative stress.
These findings suggest CoQ10 could be a valuable fertility-enhancing therapeutic strategy, particularly for older women, those with poor ovarian reserve, or those with conditions like Polycystic Ovary Syndrome (PCOS).
Background
Infertility (the inability to naturally conceive despite repeated and sustained attempts) is a growing global health concern, with 17.5% of the adult human population estimated to suffer from the condition. For women, research suggests that the primary factor determining fertility is the quality of oocytes (eggs).
Oocytes are cellular powerhouses, now known to be packed with more mitochondria than any other cell in the body. This massive energy reserve has been identified as essential for fueling oocyte maturation, fertilization, and the initial stages of embryonic development.
Unfortunately, recent research has found that as women age, the number and efficiency of these mitochondria decline. The resultant energy deficit, coupled with increased cellular damage from oxidative stress, can lead to lower-quality eggs, higher rates of chromosomal abnormalities, and reduced chances of a successful pregnancy. While fertility-assisting technologies like in vitro fertilization (IVF) have helped millions, their success rates are still fundamentally limited by the biological quality of the gametes.
Consequently, addressing the root cause of the condition (interventions that can protect and rejuvenate the oocyte at a cellular level) is essential to mitigating the growing fertility crisis and safeguarding our future generations, particularly in today’s gradually aging society.
About the study
This review aims to address the existing knowledge gap by conducting a comprehensive scientific review to synthesize the current literature on coenzyme Q10 (CoQ10) and its effects on female fertility. The review systematically examined years of existing research, from foundational laboratory studies (in vitro) on cellular mechanisms to clinical trials involving women undergoing fertility treatments (human preclinical and clinical trials).
The review’s primary objective was to determine whether and how CoQ10, a lipid-soluble compound essential for producing adenosine triphosphate (ATP), the primary energy currency of the cell, interacts with the female reproductive system. It investigates the molecule’s dual role as both an energy production facilitator and a potent antioxidant that neutralizes harmful reactive oxygen species (ROS). While CoQ10 is naturally synthesized in the body and can be obtained from dietary sources such as fatty fish and nuts, its endogenous production declines with age.
Study findings
Review findings provide promising evidence that CoQ10 directly modulates age-associated cellular challenges (ovarian aging and poor oocyte quality) by enhancing mitochondrial function. Specifically, by improving mitochondrial function, in part through key pathways such as SIRT1 and PGC-1α, CoQ10 helps restore the oocyte’s energy supply, which is crucial for its development.
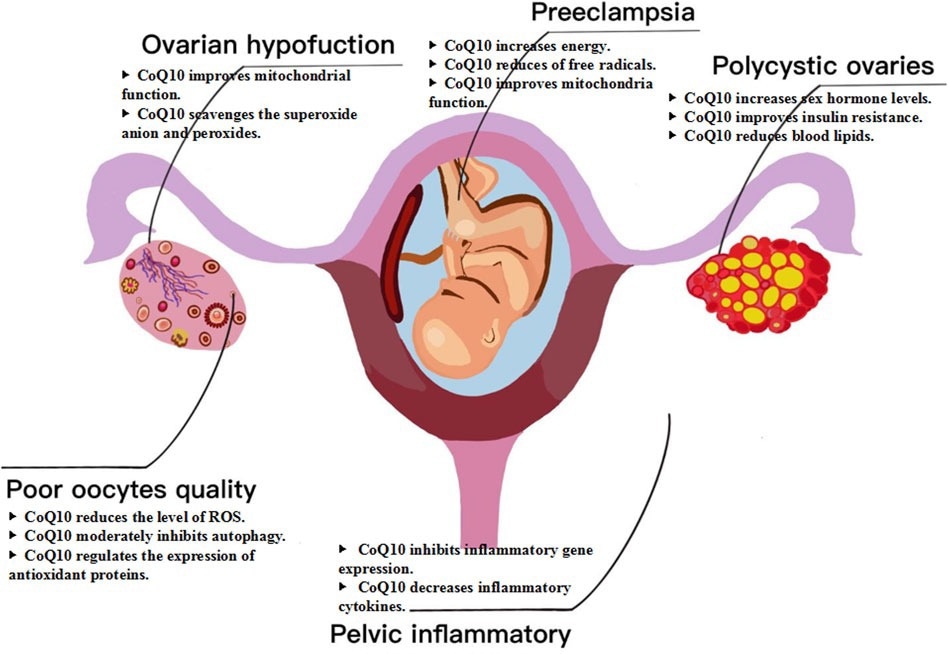
Mechanism of CoQ10 for female fertility. CoQ10 has emerged as a potential intervention for female infertility, specifically in cases related to ovarian dysfunction, poor oocyte quality, polycystic ovaries, pelvic inflammatory disease, and other factors.
For example, a key randomized controlled trial (RCT) involving young women with poor ovarian reserves demonstrated that participants who received CoQ10 supplementation (‘cases’) had a higher number of retrieved eggs, better fertilization rates, and more high-quality embryos than their control (placebo-fed) counterparts. Most notably, the rate of canceled embryo transfers due to poor embryo development dropped significantly in the CoQ10 group (22.89% to 8.33%). However, the review also critically points out that this same study, along with others, found no significant difference in the ultimate clinical pregnancy or live birth rates, suggesting that while CoQ10 improves intermediate markers, its effect on the final outcome requires more research. Additionally, the review highlights that in women with PCOS, CoQ10 supplementation has been shown to improve hormonal balance and metabolic markers.
In vitro and preclinical laboratory studies support these findings, as seen in a study wherein adding CoQ10 to the culture medium for maturing oocytes significantly improved outcomes (maturation rates increased from 48.9% to 75.7%). The paper also notes that the benefits of CoQ10 can be enhanced when used in combination with other treatments. For instance, synergistic effects were observed with Vitamin E and non-pharmacological interventions, such as transcutaneous electrical acupoint stimulation.
Finally, studies have found that CoQ10 supplementation may directly counteract ovarian aging, mitigate mitochondrial dysfunction, and reduce oocyte DNA damage. While further research is required to establish optimal, patient-specific dosages, the review notes use-case dosing has included 200 mg/day for women undergoing standard IVF and a higher dose of 600 mg/day for those with diminished ovarian reserve. The paper also cites a general human safety ceiling of 1,200 mg/day. However, gastrointestinal side effects may occur at levels above this threshold, and its safety in pregnancy and lactation has not been thoroughly validated.
Conclusions
The present review findings suggest that CoQ10 may serve as a promising therapeutic agent in (female) reproductive medicine. It highlights how CoQ10 directly targets the age-related decline in oocyte quality that underlies many cases of female infertility by boosting cellular energy production and providing critical antioxidant protection.
However, the review also underscores persistent research gaps that need to be overcome before CoQ10 interventions can enter the realm of mainstream medicine. Specifically, larger, more robust clinical trials are required to establish standardized protocols for dosage and treatment duration for different patient populations, such as those with polycystic ovary syndrome (PCOS) or premature ovarian failure. Future work should also focus on the synergistic effects of CoQ10 in combination with other therapeutic modalities to optimize treatment strategies.