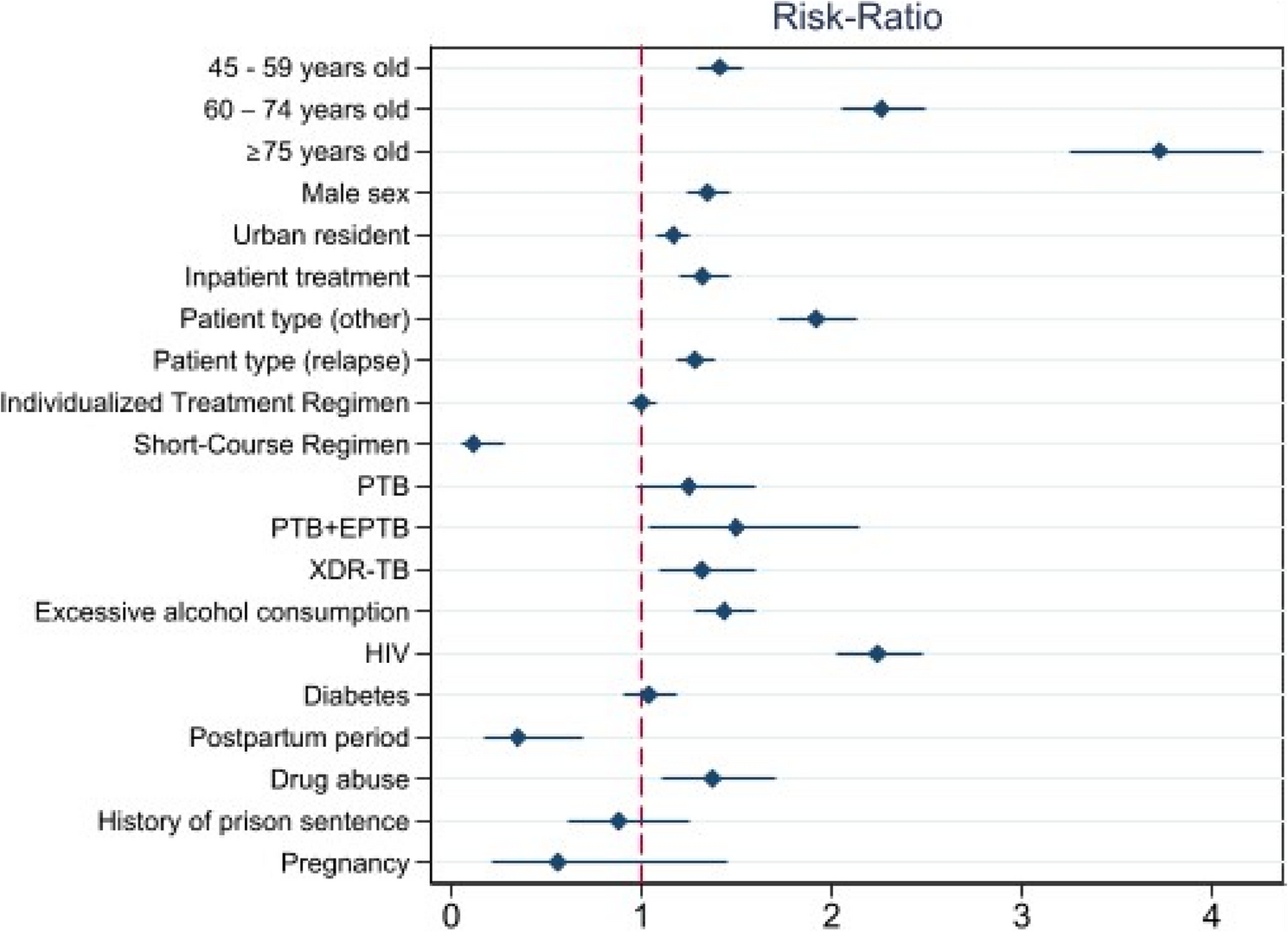
This study evaluated the effectiveness of treatment in patients with DR-TB using the nationwide TB registry in Kazakhstan, where the burden of MDR-TB is notably high. The findings of our study revealed that the overall rate of unsuccessful treatment was 18.84%, which is lower than that reported in the WHO’s global estimates [22], ranging from 27.0 to 45.0%. We found that the rate significantly increased with age, as well as for relapse and “other” patient categories, but was less likely to occur in newly treated patients.
Kazakhstan has historically ranked among the countries with the highest DR-TB burden worldwide [22, 23]. To effectively grapple with its impact, the country has introduced the National Tuberculosis Program [11], established an efficient surveillance system to monitor epidemiological metrics through local tuberculosis dispensaries [24], and aggregated data in the National Tuberculosis Registry through the Unified National Electronic Healthcare System [13]. These efforts have led to a decline in the TB burden in the country; however, DR-TB continues to be a major public health concern in the country due to the emergence of MDR-TB strains. In 2018, the WHO recommended all-oral regimens for the treatment of MDR-TB [22]. Thus, the individualized treatment regimen did not reveal any differences in our study; however, our findings were in favor of the short-course regimen and agreed with the existing evidence [25,26,27], which contributed to its recommendation by WHO. In Kazakhstan, the Central Medical Advisory Committee decides who is eligible for the short-course regimen based on the local TB protocol [15]. The bacteriological results, radiologic findings, prior treatment history, and drug availability are considered when ascertaining a regimen. Although the short-course regimen group showed encouraging findings, they should be viewed as exploratory because they are based on a small number of events in a small subgroup. Further studies are required to confirm this association.
Successful treatment was defined as being cured or having completed treatment. Thus, better tolerance and lower adverse event rates than those of other regimens could partially explain this superiority [27]. In Kazakhstan, local guidelines recommend a short-course regimen for both MDR/XDR-TB patients who are resistant to fluoroquinolones and second-line injectable agents [15]. The scheme consists of an intensive period of 4–6 Bdq (6 months) – Lfx-Cfz-Z-E-Hh-Eto and a maintenance phase of 5 Lfx-Cfz-Z-E. With a shortened complete oral treatment regimen, the total course of treatment was 9–12 months. The prolonged treatment regimen included at least five TB drugs in groups A and B.
Notably, the short-course regimen had the smallest sample size in our study, of which only 0.21% completed treatment with an unsuccessful outcome. In other regimens, the average rate was 20.00%. Hence, in a post-hoc analysis, we excluded patients for whom a short-course regimen was assigned and compared the coefficients between the two models (Supplementary Fig. 2). This resulted in the estimates for all the covariates remaining unchanged. Nevertheless, future studies should closely examine the contribution of the short-course regimen when new data on treatment outcomes become available.
The WHO recommends favoring outpatient treatment, and Kazakhstan still has a high admission rate for inpatient TB centers [5, 11]. Interestingly, our findings suggest that the risk of unsuccessful treatment is higher in inpatient settings. The unsuccessful treatment outcomes in our study were primarily driven by death and loss to follow-up. When considered separately, both were more frequent in inpatient treatment. This is most likely justified by the fact that more complicated patients were hospitalized in inpatient facilities. Although the overall figures for these metrics were in line with those from WHO estimates [28, 29], future studies should thoroughly examine these associations.
The treatment success rate was significantly lower in patients with XDR-TB. Although these cases accounted for only 2.42% of our cohort, data from a recent study on DR-TB treatment outcomes in five post-Soviet Union countries suggest that our findings are natural [30]. Furthermore, for XDR-TB, our success rate was in line with the WHO’s goal of a 75.00% success rate [31]. Similar to MDR-TB, our findings were encouraging for both global estimates [32] and Kazakhstan, which was reported recently [33].
MDR-TB continues to pose a public health crisis and threat. In 2021, there were 450,000 incident MDR-TB cases globally, and the number of such cases is expected to increase [2]. This trend requires improvements in MDR-TB control, access, and treatment approaches. Continuous monitoring of MDR-TB treatment outcomes is, therefore, essential. Such vigilance helps policymakers evaluate the effectiveness of therapeutic interventions. Although our study revealed encouraging findings in terms of treatment success, further studies are still essential, specifically exploring the role of the short-course regimen, comparing the treatment modes, the impact of patient types on treatment outcomes, and collecting real-world data on safety parameters affecting treatment outcomes.
Limitations
This study has several limitations. The major limitation was attributable to the use of secondary data that could underreport range of factors affecting treatment outcomes. For instance, underreported prevalence for some essential variables, such as HIV infection, duration of symptoms before treatment, and coexisting diseases, may affect our findings. Another limitation is that we were unable to draw clear inferences about the effectiveness of the treatment regimens, and the external validity may be weak because of the unequal distribution of participants across treatment regimens and the resistance type (MDR/XDR-TB), and exclusion of those aged less than 18 years. The lower rate of unsuccessful treatment for the short-course regimens is encouraging, but its generalizability is unclear.
Furthermore, we found that pregnancy and the postpartum state were associated with a lower risk of unfavorable treatment outcomes. This may be attributed to selection bias, with these groups representing only 0.57% and 1.85% of the total population, respectively. When sample size is small, inferences are extremely sensitive to small deviations, leading to ambiguous conclusions [34]. Hence, we may have model specification issues that require further investigation in these populations. Therefore, our findings for these categories require careful interpretation. Generalizability might be strengthened by more data on baseline resistance to other component drugs, and from prospective trials comparing short-course regimens to those of duration that is more conventional. Finally, because we used aggregated data and lacked detailed information on treatment regimens, we were unable to provide comprehensive information. Furthermore, we could not adjust for severity indicators (e.g., radiography, new vs. retreatment, and bedaquiline resistance) when comparing inpatient and outpatient treatment means and treatment outcomes in general. Therefore, our findings remain exploratory and should be interpreted with caution.