We reported a rare case of disseminated M. kansasii coinfection with T. marneffei in a patient with no prior evidence of immunodeficiency. While NTM infections represent the most frequent clinical manifestation in patients with anti-IFN-γ autoantibodies, and T. marneffei infections have been well-documented in this population, the concurrent presentation of disseminated M. kansasii and T. marneffei infections associated with anti-IFN-γ autoantibodies remains exceptionally rare in clinical literature.
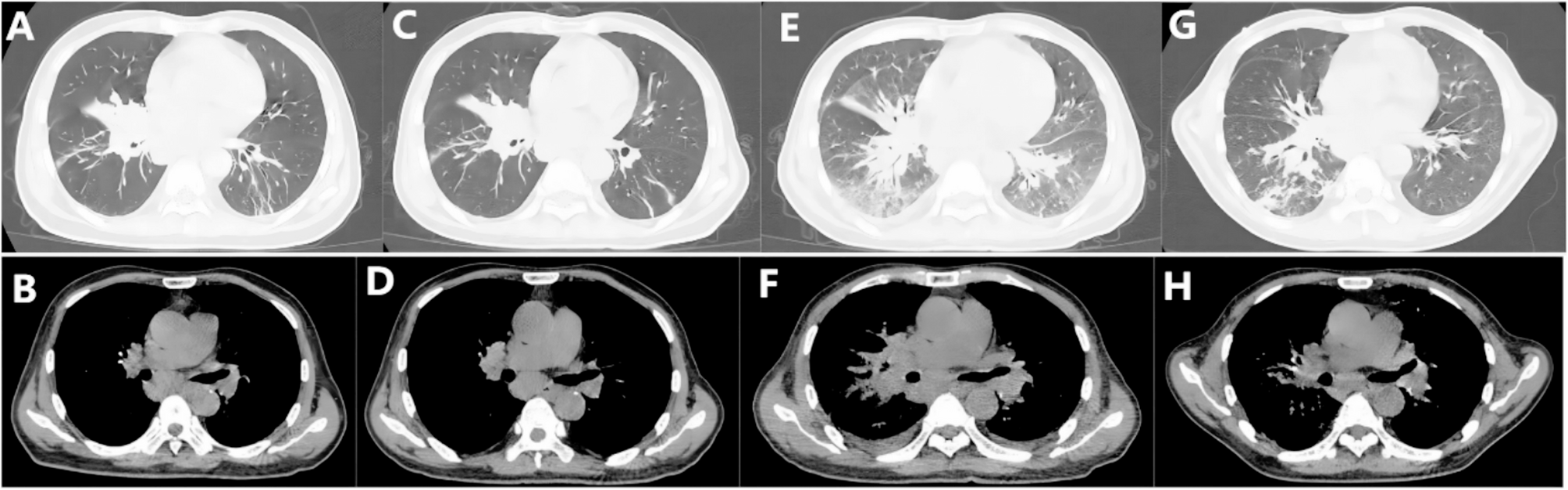
The incidence of NTM disease has shown rapid growth in recent years, emerging as a significant public health concern with substantial clinical implications [7, 8]. Disseminated NTM infections may manifest as lymphadenitis, cutaneous lesions, osteomyelitis, or hepatosplenic involvement. The clinical presentation is often nonspecific and may include constitutional symptoms (fever, night sweats, weight loss) alongside physical findings such as dermatological eruptions, lymphadenopathy, and hepatosplenomegaly, Making clinical differentiation from other infectious processes challenging. Diagnostic challenges arise from both nonspecific radiographic features and histopathological overlap with tuberculosis. While conventional bacterial culture remains the most sensitive diagnostic modality for NTM identification, certain species require specialized culture conditions including specific media, optimized temperatures, or prolonged incubation periods. In this context, mNGS has emerged as a powerful diagnostic tool, enabling rapid detection of complex and atypical pathogens within 24 h [9]. Notably, while conventional cultures failed to identify pathogens in our case, mNGS successfully detected M. kansasii within one day, underscoring the clinical value of this advanced molecular technique in the diagnosis of NTM infections.
However, the diagnostic advantages of mNGS must be balanced against its challenges. The technique‘s high sensitivity may detect clinically irrelevant nucleic acids, such as CMV or commensal microorganisms, complicating the distinction between colonization, contamination, and true infection. In immunocompromised hosts, for instance, CMV DNA detection may represent latent viral reactivation rather than active disease, explaining its presence in mNGS results without necessitating antiviral therapy. Similarly, the detection of M. kansasii by mNGS alone cannot definitively establish its pathogenic role without supporting clinical, radiological, and microbiological evidence (e.g., compatible symptoms, positive cultures, or histopathological findings). Thus, clinicians should interpret mNGS findings judiciously, incorporating the patient’s immune status, microbial burden, and corroborating diagnostic data to guide therapeutic decisions.
Disseminated M. kansasii infection remains exceptionally rare in immunocompetent populations. This underscores the importance of testing for anti- IFN-γ autoantibodies in HIV-negative patients presenting with atypical mycobacterial infections, as clinicians should maintain a high index of suspicion for this condition. IFN-γ, primarily secreted by T lymphocytes and natural killer (NK) cells, serves as a critical mediator of cellular immunity and plays a pivotal role in host defense against mycobacterial infections. First identified in 2004, anti-IFN-γ autoantibodies cause acquired adult-onset immunodeficiency (AOID) [10]. These autoantibodies neutralize IFN-γ bioactivity, impairing STAT1 phosphorylation and IL-12 production, which subsequently leads to profound Th1 cell immune dysfunction and predisposes to severe, often fatal opportunistic infections [11]. Emerging evidence suggests genetic predisposition in anti-IFN-γ autoantibody-associated AOID, particularly involving human leukocyte antigen (HLA) alleles. Pithukpakorn et al. [12] demonstrated significant associations with HLA-DRB1 and HLA-DQB1 alleles, specifically DRB1*15:01, DRB1*16:02, DQB1*05:01, and DQB1*05:02. Subsequent studies [2, 13] confirmed the involvement of DRB1*16:02 and DQB1*05:02, while co-expression of DRB1*15:02 and DQB1*05:01 may confer additional disease risk [14]. Unfortunately, technical limitations precluded HLA genotyping in our case.
Disseminated M. kansasii infection associated with anti- IFN-γ autoantibodies represents a distinct clinical entity among NTM infections. While less common than other NTM infections, this condition typically demonstrates good responsiveness to antimicrobial therapy, with most patients achieving complete recovery [6, 15]. Current Chinese guidelines recommend a 12-month regimen of isoniazid, rifampicin, ethambutol, and clarithromycin for disseminated M. kansasii infection [1]. While drug susceptibility testing of isolated strains would provide optimal guidance for antimicrobial selection, we were unable to obtain M. kansasii isolates or perform susceptibility testing due to laboratory constraints. This limitation notwithstanding, the recommended empirical regimen was initiated. The therapeutic agents may induce adverse effects, as exemplified by drug hypersensitivity reactions in our patient, along with gastrointestinal disturbances and hepatorenal toxicity, potentially compromising treatment adherence and increasing therapeutic challenges. These challenges highlight the need for optimized therapeutic approaches.
Notably, this case presented with concurrent and sequential polymicrobial infections, indicating the potential inadequacy of antimicrobial monotherapy for complete disease control. These findings underscore the need for comprehensive therapeutic strategies targeting both the infectious pathogens and underlying immune dysfunction. Rituximab, an anti-CD20 monoclonal antibody, has demonstrated clinical efficacy in reducing anti-IFN-γ autoantibody titers and restoring IFN-γ signaling pathways [16]. Alternative therapeutic options for AOID associated with anti-IFN-γ autoantibodies include cyclophosphamide, methylprednisolone, therapeutic plasma exchange, and intravenous immunoglobulin, all of which have shown varying degrees of clinical benefit in reported cases [17]. In the present case, immunomodulatory therapies were not implemented due to multiple considerations, including the high cost and unproven universal efficacy of rituximab (currently still under investigation), potential exacerbation of recurrent infections with immunosuppressive agents, and the lack of standardized treatment protocols. These clinical challenges highlight the need for further research to optimize therapeutic strategies for managing this form of immunodeficiency.
The patient had recurrent high fever after anti-NTM treatment, and T. marneffei was finally found in the blood culture. The presented case of cellular immunodeficiency demonstrates characteristic susceptibility to both mycobacterial infections and opportunistic fungal pathogens, particularly T. marneffei, consistent with previous reports of such co-infections in immunocompromised hosts [2, 13, 18]. T. marneffei infection in this context warrants particular attention given its emerging recognition as a sentinel infection in patients with impaired cellular immunity, especially those with anti-IFN-γ autoantibodies. Its detection should prompt comprehensive immunological evaluation.
T. marneffei represents a significant opportunistic fungal pathogen predominantly affecting immunocompromised individuals, serving as the most prevalent opportunistic mycosis in HIV-positive populations across Southeast Asia and southern China [19]. This thermally dimorphic fungus primarily targets the mononuclear phagocyte system, with predilection for the lungs, skin, lymphatic tissues, and bone marrow. In HIV-negative patients, T. marneffei infection presents with diverse and nonspecific clinical manifestations that may include multiorgan dysfunction, systemic osteolytic lesions, hepatosplenomegaly, and generalized lymphadenopathy, often mimicking tuberculosis, pneumonia, lymphadenitis, or metastatic malignancies. Definitive diagnosis relies on isolation of T. marneffei from clinical specimens such as sputum, blood, or bronchoalveolar lavage fluid through microbiological culture.
The diagnosis of T. marneffei infection remains challenging due to the time-consuming nature of fungal culture (typically requiring 7–10 days) and the notably low culture positivity rates observed in HIV-negative individuals. Emerging evidence suggests that anti-IFN-γ autoantibody positivity may predispose HIV-negative patients to severe T. marneffei infections. When such patients exhibit progressive clinical deterioration despite appropriate therapy, clinicians should maintain a high index of suspicion for concurrent opportunistic infections, particularly talaromycosis. In cases presenting with multisystem involvement, comprehensive diagnostic evaluation including simultaneous biopsies from multiple affected sites may be warranted to characterize the pathological nature of the lesions.
The therapeutic approach for T. marneffei infection in HIV-negative patients currently mirrors that for HIV-positive individuals due to the absence of specific treatment guidelines. Current recommendations adapted from HIV-positive protocols [20] advocate initial amphotericin B therapy followed by consolidation with either voriconazole or itraconazole, with treatment duration traditionally guided by CD4 + T-lymphocyte counts in immunocompromised hosts. Patients with anti-IFN-γ autoantibodies require prolonged antifungal courses due to persistent immunodeficiency to prevent disease recurrence. The coexistence of M. kansasii infection in our patient substantially increased therapeutic complexity. Concurrent administration of amphotericin B with antimycobacterial agents (particularly rifampin) presents three major challenges: cytochrome P450-mediated drug interactions, cumulative nephrotoxicity, and overlapping adverse effects that may impair treatment tolerance. Furthermore, the extended treatment durations required for both infections – typically ≥ 12 months for NTM and ≥ 6 months for T. marneffei – pose unique adherence challenges. This dual-pathogen scenario necessitates individualized therapeutic drug monitoring, frequent renal function assessments, and careful balancing of microbial eradication against medication toxicity.
Patients with anti-IFN-γ autoantibodies frequently develop complex clinical presentations characterized by concurrent or sequential polymicrobial opportunistic infections, posing significant diagnostic challenges. Early implementation of mNGS facilitates timely pathogen identification and therapeutic optimization, which may improve clinical outcomes. However, the absence of established treatments for the underlying immunodeficiency highlights the critical need for developing evidence-based management guidelines.