It is recognized that HBV is the most important cause of liver failure [19]. According to research [9], more than 80% of liver failure in China is related to HBV infection. In the case of liver failure in HB, severe impairment of liver function, gastrointestinal symptoms, coagulation disorders and severe jaundice will occur [30], with a mortality rate up to 50% or even close to 90% [18]. It has been confirmed in previous studies that there is a close correlation between impaired immune function and viral replication in HBV patients [23], in which T lymphocytes play an important role. The dysfunction of T lymphocyte subsets is considered as an important cause of enhanced HBV replication [11]. Currently, the most prevalent clinical form of liver failure in China, ACLF is closely associated with HBV infection. Moreover, it is believed by most scholars that ACLF has a certain correlation with host immune response disorders caused by acute intrahepatic and extrahepatic events [14]. Due to no effective treatment for ACLF at present, effectively improving the body’s immune function, regulating the liver and kidney function parameters, and reducing the damage of excessive immune response to hepatocytes are the key to prevention.
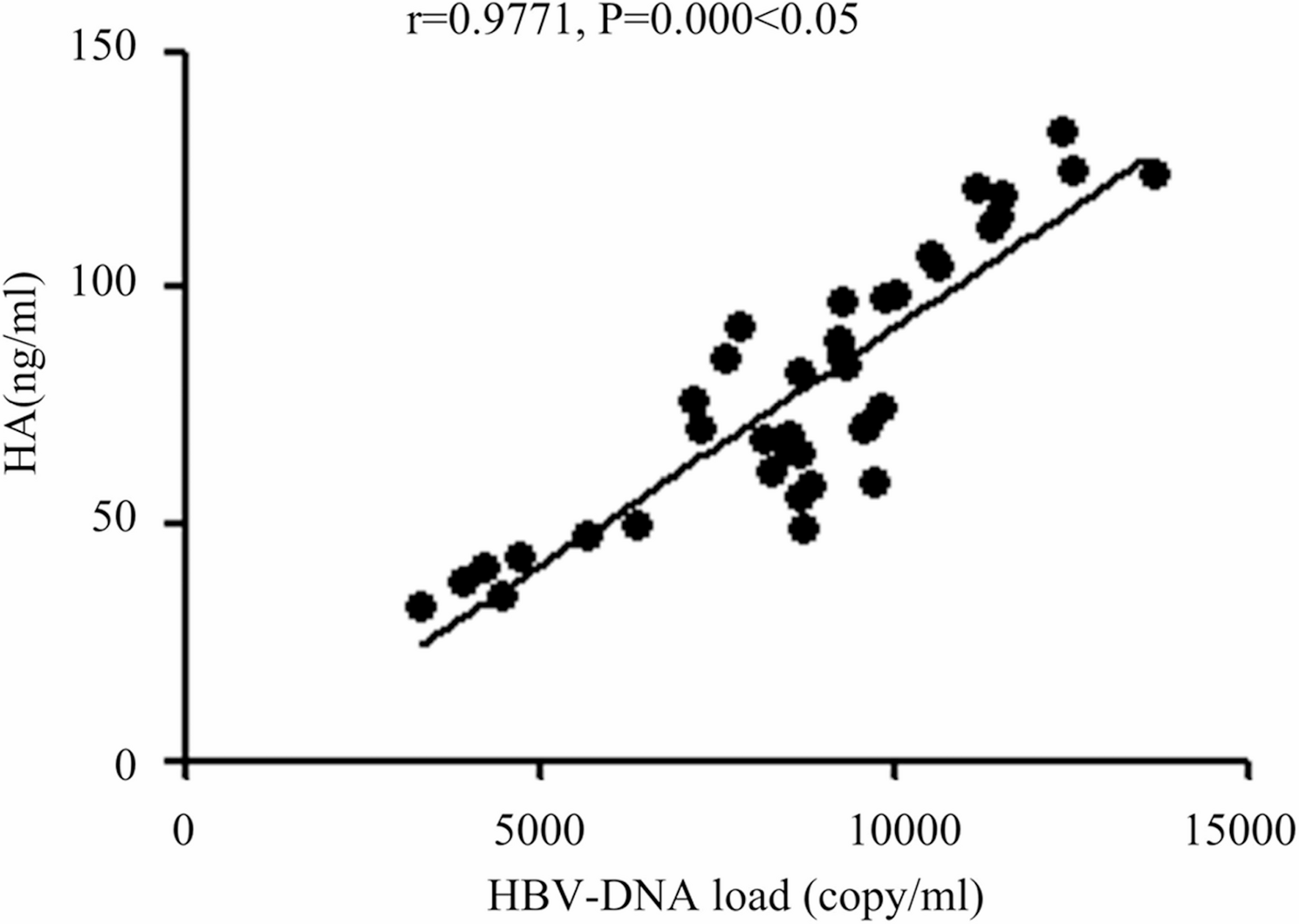
In this study, patients with HB and those with HBV-ACLF had their clinical features compared. Antiviral treatment (both ETV and TDF are nucleoside analogs) has been shown to effectively suppress HBV replication, thereby significantly reducing HBV-DNA levels. This may influence the HBV-DNA levels observed in the study. However, given that the proportions of patients receiving each drug type and the median treatment durations were comparable between the two groups (P > 0.05), the impact of antiviral therapy on HBV-DNA levels is likely similar between the two groups, thereby minimizing bias in the inter-group comparisons. It was found that observation group had significantly higher HBV-DNA load and levels of HA, PCIII, ALT, AST, TBIL, BUN, Cr, hs-CRP and TNF-α than control group, while the CD4+ percentage and CD3+ percentage in observation group were obviously lower than control group. HBV-ACLF patients exhibited significantly reduced proportions of peripheral blood CD3 + and CD4 + T cells, indicative of a systemic immunosuppressive state. HBV infection has been shown to impair mitochondrial oxidative phosphorylation (OXPHOS) in CD3 + T cells, suppressing ATP production and consequently weakening their proliferative and effector functions [24]. This metabolic reprogramming may exacerbate the immunosuppressive microenvironment through dysregulation of the AMPK/mTOR pathway [31]. Persistent HBV replication can also induce elevated expression of exhaustion markers (e.g., PD-1, Tim-3) in CD8 + T cells, leading to impaired cytotoxic activity [1]. Although this study did not directly assess CD8 + subsets, the observed decline in CD3 + T cells may reflect CD8 + T cell apoptosis or functional exhaustion, thereby compromising viral clearance and aggravating hepatocyte injury [2]. Furthermore, an increased proportion of regulatory T cells (Tregs) in HBV-ACLF patients may suppress effector T cell activity and promote immune tolerance [20]. This imbalance could be amplified by elevated IL-10 and TGF-β secretion, further reinforcing immunosuppression within the hepatic microenvironment. This study detected markedly elevated serum TNF-α and hs-CRP levels in HBV-ACLF patients, underscoring the central role of inflammatory cascades in disease progression. TNF-α induces hepatocyte apoptosis via caspase-3 pathway activation [27] and, upon binding to TNFR1, activates hepatic stellate cells, accelerating fibrogenesis [22]. The findings above show that in HBV-ACLF, the HBV-DNA load in vivo obviously rises, liver cirrhosis is obvious, liver and kidney function parameters are severely damaged, the body’s inflammatory response is remarkable, and the immune ability of T lymphocytes evidently declines. This study found that HBV-DNA load was negatively correlated with the proportion of CD3 + T cells, but positively correlated with the levels of hs-CRP and TNF-α, suggesting that there was a vicious circle among virus replication, inflammatory reaction and immunosuppression. High-level replication of HBV-DNA activates monocytes and macrophages through TLR2/MyD88/NF-κB pathway, releasing IL-6 and TNF-α [28], further inhibiting T cell function. Besides, the results of the 1-month follow-up revealed that the proportions of infection, gastrointestinal bleeding, hepatic encephalopathy, electrolyte disturbance and ascites in observation group were evidently larger than control group, which suggest that complications occur more easily in HBV-ACLF, with a higher risk. Finally, the univariate and multivariate logistic regression analyses of risk factors for HBV-ACLF revealed that the pathophysiology of HBV-ACLF was associated with abnormalities in peripheral blood T lymphocyte percentage, inflammatory factors, hepatic function, renal function, liver cirrhosis indexes, and HBV-DNA load. The increases in the HBV-DNA load, liver cirrhosis indexes, hepatic function indexes, renal function indexes and inflammatory factors were independent risk factors, while the increase in the peripheral blood T lymphocyte percentage was the protective factor for the pathogenesis of HBV-ACLF. This study is consistent with previous reports on ACLF systemic inflammation and T cell depletion [13, 29], but it expands the existing cognition. It is found that the levels of HA and PCIII not only reflect the degree of liver fibrosis at baseline, but also increase dynamically in the progress of ACLF, suggesting that they have dual functions as fibrosis markers and predictors of acute decompensation, which provides new theoretical basis and clinical reference for accurate diagnosis and individualized treatment of HBV-ACLF.
In clinic, ACLF refers to a group of clinical syndromes (coagulation disorders, severe jaundice, massive ascites and even hepatic encephalopathy) occurring within a short period of time after acute liver injury based on chronic liver disease [32, 33]. HBV infection is considered as the most important cause of ACLF [7]. In this study, it was found that ACLF was mainly related to the HBV-DNA load. With the decline in the body’s immune function and the destruction of balance between HBV and body immunity [3], T lymphocytes will specifically attack virus-infected hepatocytes [32, 33], and promote the activation of cellular and humoral immunity, thereby further aggravating the death of infected hepatocytes, the body’s inflammatory response and hepatic-renal dysfunction [15]. Our study identified four independent risk factors (HBV-DNA load, hs-CRP, TNF-α, and CD3 + T cell percentage) that provide novel insights for precision management of HBV-ACLF. These biomarkers enable early identification of high-risk patients, guiding intensive monitoring protocols (including daily liver function tests) and initiating prophylactic anti-infective therapy. The dynamic changes in these biomarkers offer real-time prognostic value for monitoring disease progression. These findings establish a theoretical foundation for innovative therapeutic approaches in HBV-ACLF. For addressing cytokine storms, dual inhibition of the TNF-α/IL-6 signaling axis may alleviate hepatocyte damage while preventing excessive immunosuppression. Based on the critical risk factors for HBV-ACLF identified in this study, the following treatment strategies are recommend: for patients with high viral loads, prompt initiation of potent antiviral therapy to rapidly suppress viral replication and reduce immune-mediated hepatocyte injury; for patients with persistently low CD3 + T cell percentages and elevated TNF-α levels, immunomodulatory therapies targeting T cell exhaustion or inflammatory signaling pathways should be explored to improve the immune microenvironment. These evidence-based strategies integrate risk stratification with personalized treatment approaches to guide clinical decision-making.
In summary, this study provides a multidimensional characterization of HBV-ACLF by integrating virological, immunological, and fibrotic markers. The dynamic changes of HA and PCIII during ACLF progression and their synergistic predictive value with HBV-DNA and T-cell subsets offer novel insights for risk stratification. These findings advance the field by proposing a composite biomarker panel that may enhance early detection and prognostic evaluation of HBV-ACLF, thereby establishing a foundation for targeted therapeutic strategies. However, several limitations should be acknowledged: (1) The single-center design with relatively small sample size may limit generalizability, necessitating validation through multicenter, large-scale cohorts; (2) The cross-sectional design precludes causal inference regarding the relationships among HBV-DNA load, inflammatory markers, and T-lymphocyte profiles with ACLF onset, warranting further longitudinal monitoring; (3) The lack of antiviral treatment data may confound the association between viral load and outcomes, requiring future studies with comprehensive treatment records and stratified analyses; (4) The 1-month follow-up period precluded assessment of long-term outcomes (e.g., 90-day mortality), necessitating extended observation periods; (5) Liver fibrosis markers (HA, PCIII) may be confounded by acute liver injury without histological or imaging confirmation of chronic fibrosis, requiring additional validation with these modalities. While the exclusion of elderly patients and those with comorbidities enhanced internal validity, it may limit generalizability to real-world populations, suggesting the need for broader inclusion criteria in future studies. Notwithstanding these limitations, this study provides valuable biomarker clues for early warning of HBV-ACLF and establishes an important foundation for further mechanistic investigations.