Breast cancer continues to impact millions worldwide, and for many, the diagnosis involves a hormone receptor-positive subtype.1 These cancers rely on estrogen or progesterone to grow, using hormone receptors on their cells to drive tumor development.
For decades, tamoxifen has been a cornerstone treatment. It works by blocking these hormone receptors and disrupting the downstream signaling that supports cancer growth.
But not all patients respond well. A significant number develop resistance to tamoxifen, leaving clinicians with fewer treatment options and unresolved questions.2
Now, new research is offering some insight. Scientists have identified that the loss of a tumor-suppressor protein called FRMD8 may contribute to this resistance. When FRMD8 is missing, the tumor environment begins to change in ways that appear to reduce the drug’s effectiveness.3
This finding was made possible thanks to advanced imaging and analysis tools from TissueGnostics. These technologies allowed the research team to examine breast tissue with remarkable precision—revealing subtle changes that had previously gone undetected, and helping to clarify how resistance to tamoxifen can develop.
Measuring key markers for tamoxifen resistance
Cancer cells don’t exist in isolation—they’re constantly interacting with the surrounding tissue, immune cells, and a complex network of signaling molecules. Together, these elements make up the tumor microenvironment, which plays a critical role in how cancers grow, adapt, and respond to treatment.4
To truly understand what drives tumor progression, scientists need to study this environment as a whole, not just as isolated components.
In this study, the researchers explored how the loss of FRMD8 affects the tumor microenvironment.3 They focused on three key markers:
- ERα (Estrogen Receptor alpha): The most commonly expressed hormone receptor in breast tumors
- PR (Progesterone Receptor): Encoded by a target gene of ER
- CK8 (Cytokeratin 8): A marker of mammary luminal epithelial cells
These markers were analyzed in the tissue surrounding the tumor to assess whether FRMD8 loss alters the broader microenvironment, not just the tumor cells themselves.
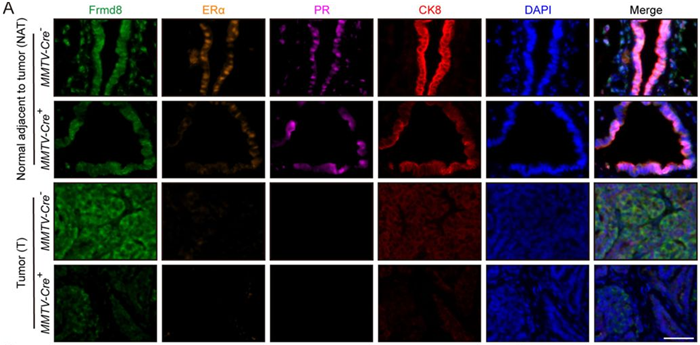
Figure 3A. Representative multiplex immunofluorescence images of tumor tissues and tissues adjacent to tumor from MMTV-Cre–; Frmd8fl/fl; PyMT and MMTV-Cre+; Frmd8fl/fl; PyMT mice. Scale bar, 50 μm. Image Credit: Adapted from Loss function of tumor suppressor FRMD8 confers resistance to tamoxifen therapy via a dual mechanism by Wu et al, 2025
Hormone receptor markers play a key role in guiding treatment decisions for breast cancer. When their expression drops, there are fewer targets for drugs like tamoxifen, raising the likelihood that the treatment may be less effective.
In this study, researchers used tissue mapping to visualize these markers and found that CK8-positive epithelial cells in the mammary tissue of FRMD8-deficient mice expressed significantly lower levels of hormone receptors compared to control mice. Notably, in young FRMD8-deficient mice, they observed reduced expression of ERα in both normal breast tissue and areas of atypical hyperplasia.
Taken together, the findings suggest that the loss of FRMD8 leads to decreased expression of both ERα and PR in mammary tissues.3
Loss of FRMD8 contributes to tamoxifen resistance
FRMD8 plays a key role in maintaining healthy tissue architecture and regulating cell signaling. This study found that when FRMD8 is absent, levels of ERα and PR drop significantly in the tissue surrounding mammary tumors—a shift that may help explain why some tumors become resistant to tamoxifen.3
Although the full biological mechanism is still being studied, early evidence points to FRMD8’s influence on both the production and stability of the hormone receptors tamoxifen targets.
As part of their research, the team investigated the FRMD8/FOXO3A regulatory axis and discovered that silencing FRMD8 led to a sharp decrease in FOXO3A, a transcription factor involved in ERα expression. They also found that FRMD8 appears to stabilize ERα protein by protecting it from degradation.
This connection between FRMD8 loss and treatment resistance could certainly become a valuable tool in clinical practice, helping identify patients at higher risk of therapy failure and allowing for more tailored treatment strategies.
Advanced imaging and analysis in cancer research
TissueGnostics’ advanced imaging technology enabled in-depth visualization of the tumor microenvironment, demonstrating how powerful cutting-edge imaging tools can help reveal the hidden drivers of treatment resistance.
The researchers used the TSA kit from TissueGnostics to amplify the fluorescent signals, making each marker clearly visible.
The use of multiplex immunofluorescence (mIF) allowed the research team to simultaneously detect several different proteins in the same piece of tissue, meaning it was possible to confidently detect ERα, PR, and CK8 expression between samples both with and without the presence of FRMD8.
Tissues were then scanned with the TissueFAXS automated slide scanning platform.
TissueFAXS can accommodate imaging up to eight fluorescent channels, delivering high-resolution images of whole slides. This imaging capability provided the researchers with a full, accurate visual record of where each protein appeared across the entire tissue sample.
The StrataQuest image analysis software was then used to analyze the scanned images at the single-cell level, allowing the researchers to measure protein expression and pinpoint the arrangement of ERα, PR, and CK8, as well as compare the levels of each marker in FRMD8-deficient or wild-type mice models.3
The future of breast cancer research
This study represents an important step forward in understanding how FRMD8 loss contributes to tamoxifen resistance. Additional research is needed in order to explore how this knowledge could lead to new interventions and to validate these findings in larger patient groups, but the implications of this work are promising.3
Understanding the tumor microenvironment and the impact of molecules like FRMD8 on this will continue to be a key tool in ongoing efforts to treat breast cancer.
The study presented here highlights the complexity of the tumor microenvironment as well as technology’s value in helping to understand this. Advanced imaging tools will continue to play a pivotal role in these types of studies, ultimately leading to a reduction in global breast cancer-related deaths.
This work also highlights the value of advanced imaging tools in uncovering currently hidden aspects of disease. For example, technologies like those from TissueGnostics are helping researchers gain insights that were historically out of reach, including revealing the subtle dynamics of breast cancer tissue at the cellular level.
TissueGnostics offers a range of multiplex immunofluorescence solutions, including the TissueFAXS imaging platform, the TSA Kit (currently available for the Chinese market), and StrataQuest image analysis software. These solutions provide precise, high-quality data designed to advance research and support new insights in cancer biology.
References and further reading
- WHO (2025). Breast cancer. (online) World Health Organization. Available at: https://www.who.int/news-room/fact-sheets/detail/breast-cancer.
- Maselli, A., et al. (2019). Autoantibodies Specific to ERα are Involved in Tamoxifen Resistance in Hormone Receptor Positive Breast Cancer. Cells, (online) 8(7), pp.750–750. https://doi.org/10.3390/cells8070750.
- Wu, W., et al. (2025). Loss function of tumor suppressor FRMD8 confers resistance to tamoxifen therapy via a dual mechanism. (online) https://doi.org/10.7554/elife.101888.2.
- Anderson, N.M. and Simon, M.C. (2020). The Tumor Microenvironment. Current Biology, 30(16), pp.R921–R925. https://doi.org/10.1016/j.cub.2020.06.081.
- Super User (2024). Contextual Tissue Cytometer | Image Processing Solution. (online) TissueGnostics . Available at: https://tissuegnostics.com/products/contextual-image-analysis/strataquest.
About TissueGnostics
TissueGnostics (TG) is an Austrian company focusing on integrated solutions for high content and/or high throughput scanning and analysis of biomedical, veterinary, natural sciences, and technical microscopy samples.
TG has been founded by scientists from the Vienna University Hospital (AKH) in 2003. It is now a globally active company with subsidiaries in the EU, the USA, and China, and customers in 30 countries.
TissueGnostics portfolio
TG scanning systems are currently based on versatile automated microscopy systems with or without image analysis capabilities. We strive to provide cutting-edge technology solutions, such as multispectral imaging and context-based image analysis as well as established features like Z-Stacking and Extended Focus. This is combined with a strong emphasis on automation, ease of use of all solutions, and the production of publication-ready data.
The TG systems offer integrated workflows, i.e. scan and analysis, for digital slides or images of tissue sections, Tissue Microarrays (TMA), cell culture monolayers, smears, and other samples on slides and oversized slides, in Microtiter plates, Petri dishes and specialized sample containers. TG also provides dedicated workflows for FISH, CISH, and other dot structures.
TG analysis software apart from being integrated into full systems is fully standalone capable and supports a wide variety of scanner image formats as well as digital images taken with any microscope.
TG cooperations
TG continuously cooperates with research groups and other companies in the industry to provide novel tools and applications to its customers.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.