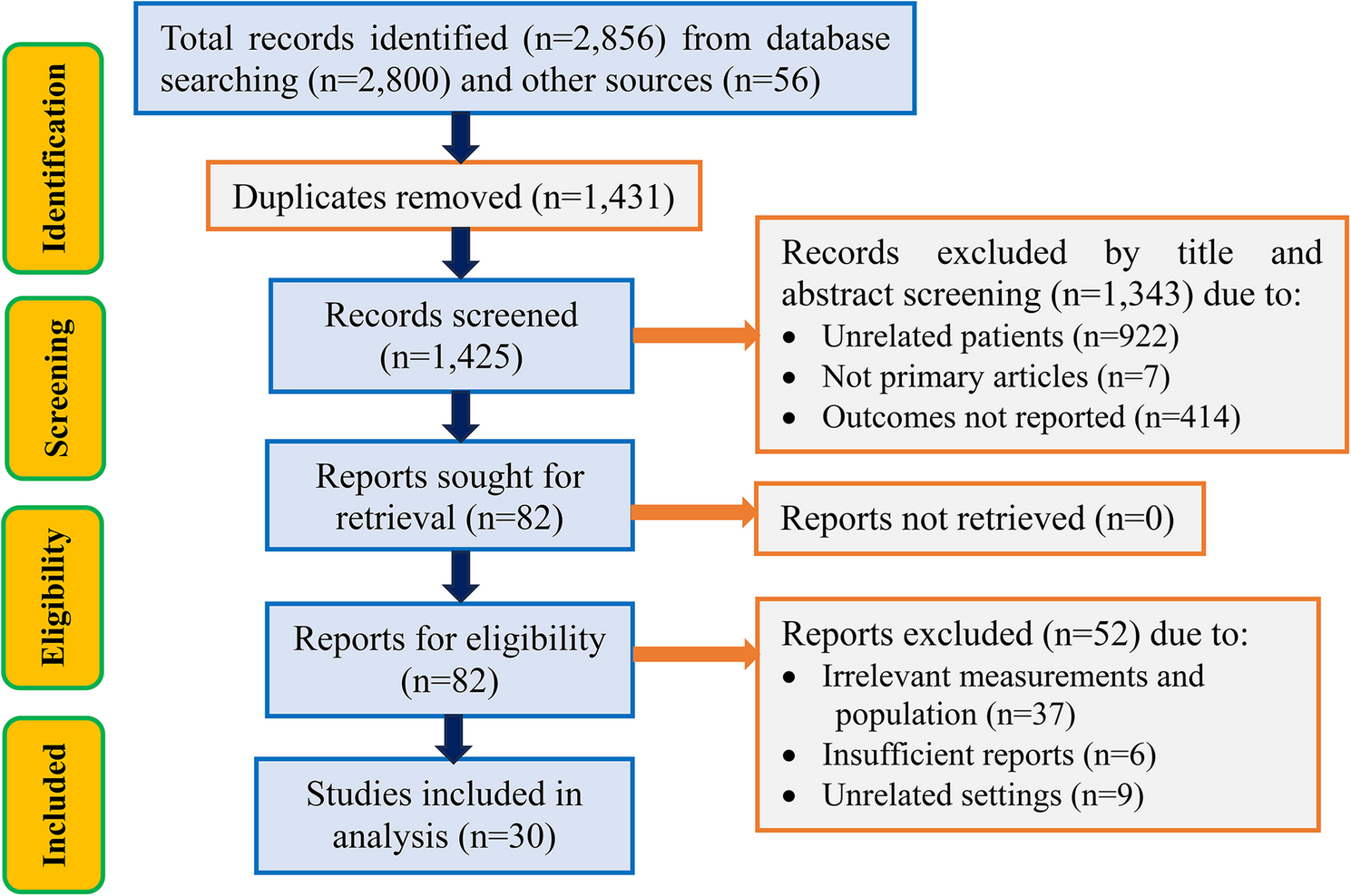
Search results
A total of 2,856 records were identified from all databases and other sources, followed by 1,431 duplication removals. Out of the 1,425 records remaining after duplicate removal, 1,343 were excluded following title and abstract screening. Subsequently, 82 reports were found eligible for further evaluation, followed by excluding 37 reports due to irrelevant measurements and population, 6 due to insufficient reports, and 9 due to unrelated settings. Finally, 30 studies that met the eligibility requirements for the review were included in the analysis (Fig. 1).
PRISMA flow chart for the studies screened, reviewed, and included
Characteristics of included studies
Data were retrieved from thirty studies published between 2007 and 2023 in eleven sub-Saharan African countries [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44] (Fig. 2).
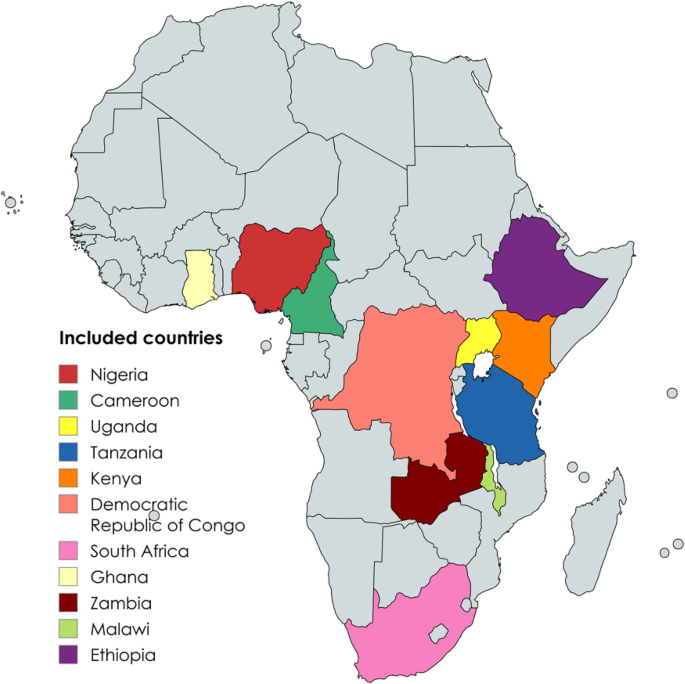
Map of Africa displaying the countries included in the study [created with https://www.mapchart.net/africa.html]
Based on continental subregions, three studies were conducted in Central [17, 30, 38], twelve in Eastern [16, 18, 19, 21, 22, 25, 32, 33, 36, 37, 40, 44], nine in Southern [20, 23, 27, 28, 31, 35, 41,42,43], and six in Western Africa [15, 24, 26, 29, 34, 39]. Specifically, two of the three studies in central Africa were conducted in the DRC [17, 30] while one is in Cameroon [38]. Of the twelve studies from the Eastern Africa region, seven were conducted in Ethiopia [16, 18, 19, 22, 36, 37, 40], two in Uganda [32, 44], two in Kenya [21, 33], and one in Tanzania [25]. Furthermore, within the Southern Africa region, South Africa [23, 31, 41, 42] and Malawi [20, 27, 28, 43], each contributed four of the nine studies from, while Zambia [35] contributed one. From the Western Africa region, four studies were from Nigeria [15, 24, 26, 29] followed by two studies from Ghana [34, 39]. Overall, a total of 13,406 HIV-TB co-infected children were included in the final analysis, with the samples ranging from 50 to 5991 (Table 2)
Pooled estimate of mortality rate
The pooled estimate of mortality rate among HIV-TB co-infected children was 15.89% (95% CI: 13.62, 18.17) with a significant heterogeneity (I2 = 92.1%, p < 0.001) (Fig. 3).
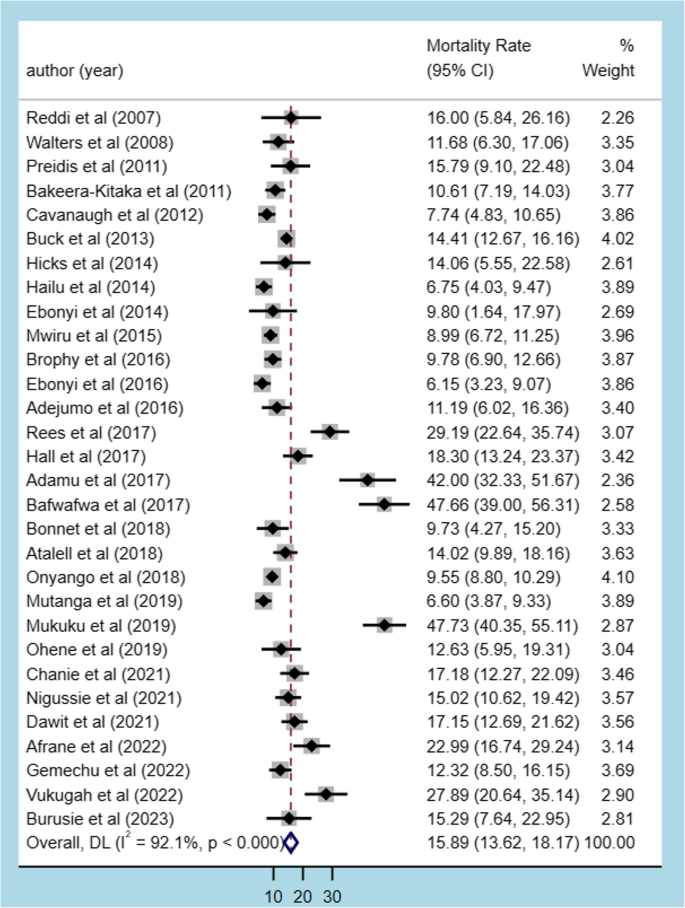
The forest plot for mortality among HIV-TB co-infected children in Africa
Subgroup analysis
Subgroup analysis was conducted using the African region, country, sampling technique, sample size, and study design. Across the African region, mortality rates varied markedly, with Central Africa having the highest mortality rate (41.00%, 95% CI: 27.62–54.37) and significant heterogeneity (I² = 88.8%, p < 0.001), while Eastern Africa recorded the lowest (11.33%, 95% CI: 9.63–13.04) with significant heterogeneity (I² = 72.5%, p < 0.001). Median mortality rates also reflected this variation, ranging from 11.50% (IQR: 5.68) in Eastern Africa to 47.70% (IQR: 9.92) in Central Africa. Heterogeneity was consistently high across subregions (I² = 72.5% − 92.2%). At the country level, mortality rates ranged from 6.60% (95% CI: 3.87–9.33) in Zambia to 47.69% (95% CI: 42.08–53.31) in the Democratic Republic of Congo (DRC). Low heterogeneity was observed in South Africa (I² = 5.6%, p = 0.365), Uganda (I² = 0.0%, p = 0.790), Kenya (I² = 27.9%, p = 0.239), and DRC (I² = 0.0%, p = 0.990), indicating consistent mortality estimates in these settings, while Nigeria (I² = 93.9%, p < 0.001), Malawi (I² = 89.8%, p < 0.001), Ethiopia (I² = 77.8%, p < 0.001), and Ghana (I² = 79.7%, p = 0.027) showed substantial between-study variability. By sampling technique, the probability technique yielded the highest mortality rate (16.50%, 95% CI: 14.05–18.96) with significant heterogeneity (I² = 92.9%, p < 0.001), while the non-probability technique had a lower rate (11.26%, 95% CI: 8.33–14.18) and no heterogeneity (I² = 0.0%, p = 0.698). In terms of study design, cross-sectional studies reported the highest mortality rate (19.66%, 95% CI: 11.08–28.24) with high heterogeneity (I² = 96.1%, p < 0.001), followed by cohort studies (14.67%, 95% CI: 12.52–16.83; I² = 88.8%, p < 0.001) (Table 3)
Meta-regression
To assess the likely sources of heterogeneity across studies, meta-regression analysis was performed using sample size, publication year, and duration of study follow-up. Accordingly, in the unadjusted meta-regression model, all variables did not significantly predict mortality rate heterogeneity across the studies. After the multivariable meta-regression model was fitted, the sample size did not significantly predict mortality rate heterogeneity across studies. However, the publication year and duration of the study significantly predicted mortality heterogeneity across the studies (Table 4)
Publication bias
Initially, the funnel plot was found to be asymmetrical, with fewer studies located at the left of the funnel and the majority of the studies dispersed on the right side of the funnel, suggesting the presence of publication bias (Fig. 4). This visual inspection was further tested using Egger’s regression and similarly showed evidence of the small-study effects (p-value < 0.001). Also, Begg’s correlation test revealed evidence of publication bias (p-value < 0.001).
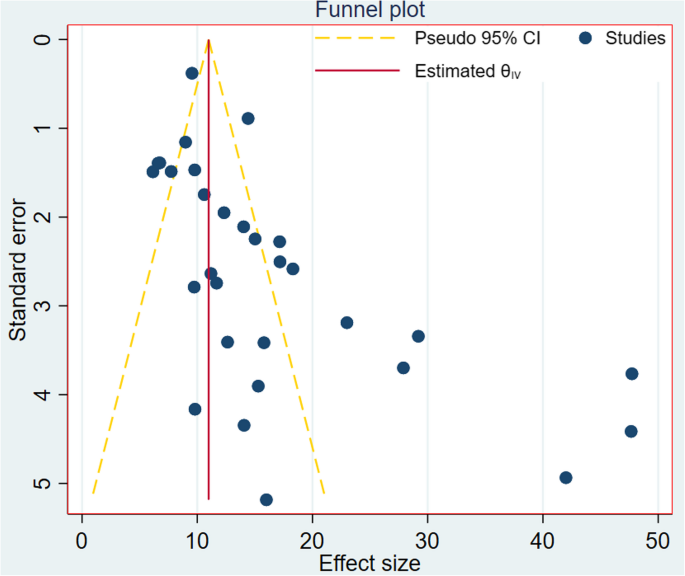
Funnel plot to display publication bias among the included studies
Factors associated with the mortality rate
A total of eight studies [15,16,17,18,19,20, 36, 37] reported at least one factor associated with the mortality rate among HIV-TB co-infected children. Accordingly, sex of the child, residence, anemia, CPT, IPT, site of TB, WHO clinical stage, initial ART regimen change, ART failure, weight for age, height for age, presence of opportunistic infections (OIs), immunosuppression, and ART adherence were found to be the factors reported by the included studies. Consequently, the POR estimates revealed that the place of residence, anemia, CPT, site of TB, immunosuppression, and ART adherence were found to display significant associations with mortality rate.
Subsequently, HIV-TB co-infected children Living in rural areas had 50% higher odds of mortality compared with their urban counterparts (POR = 1.5, 95%CI: 1.12, 1.90, I2 = 0.6%). Furthermore, the odds of mortality in children with anemia were more than seven times higher than those without anemia (POR = 7.41, 95%CI: 2.20, 12.61, I2 = 99.6%). Besides, children diagnosed with extrapulmonary TB (EPTB) had nearly six times higher odds of mortality compared with those with pulmonary TB (PTB) (POR = 5.67, 95%CI: 1.68, 9.66, I2 = 99.49%). Additionally, having severe/advanced immunosuppression was found to increase the odds of mortality by nearly six times compared to mild/moderate immunosuppression condition (POR = 5.82, 95%CI: 1.55, 10.08, I2 = 98.96%). Similarly, poor/fair adherence to ART appears to have ten times the odds of mortality than good adherence to ART (POR = 10.17, 95%CI: 3.52, 16.82, I2 = 99.70%). However, CPT was uncovered to be a protective factor against the mortality of HIV-TB co-infected children, resulting in a 62% reduction in mortality (POR = 0.38, 95%CI: 0.02, 0.73, I2 = 0.0%) (Table 5)