Study design and setting
This retrospective cohort study was conducted at Mouwasat Hospital, a tertiary care center in the Eastern Province of Saudi Arabia. We analyzed data from pediatric patients diagnosed with either Kawasaki Disease (KD) or Multisystem Inflammatory Syndrome in Children (MIS-C) between January 2020 and December 2023. The study protocol received approval from the institutional review board of Mouwasat Hospital (approval number (approval number#2023-D006), and written informed consent was obtained from all participants’ parents or legal guardians. The research protocol was conducted in accordance with the Declaration of Helsinki.
Study population and case definition
We enrolled pediatric patients aged 0–14 years presenting with febrile illness lasting three or more days, who met diagnostic criteria for either KD or MIS-C in Children. For KD diagnosis, we adhered to the American Heart Association guidelines, requiring fever for ≥ 5 days plus four of the five principal clinical criteria (bilateral non-exudative conjunctivitis, oropharyngeal changes, polymorphous exanthem, peripheral extremity changes, and cervical lymphadenopathy) or fewer criteria in the presence of coronary artery abnormalities. MIS-C diagnosis followed World Health Organization criteria, requiring fever, elevated inflammatory markers, multi-system involvement, and evidence of SARS-CoV-2 infection or exposure. We excluded patients with alternative diagnoses that could explain their presentation or those with incomplete clinical data [8, 9].
Clinical assessment and data collection
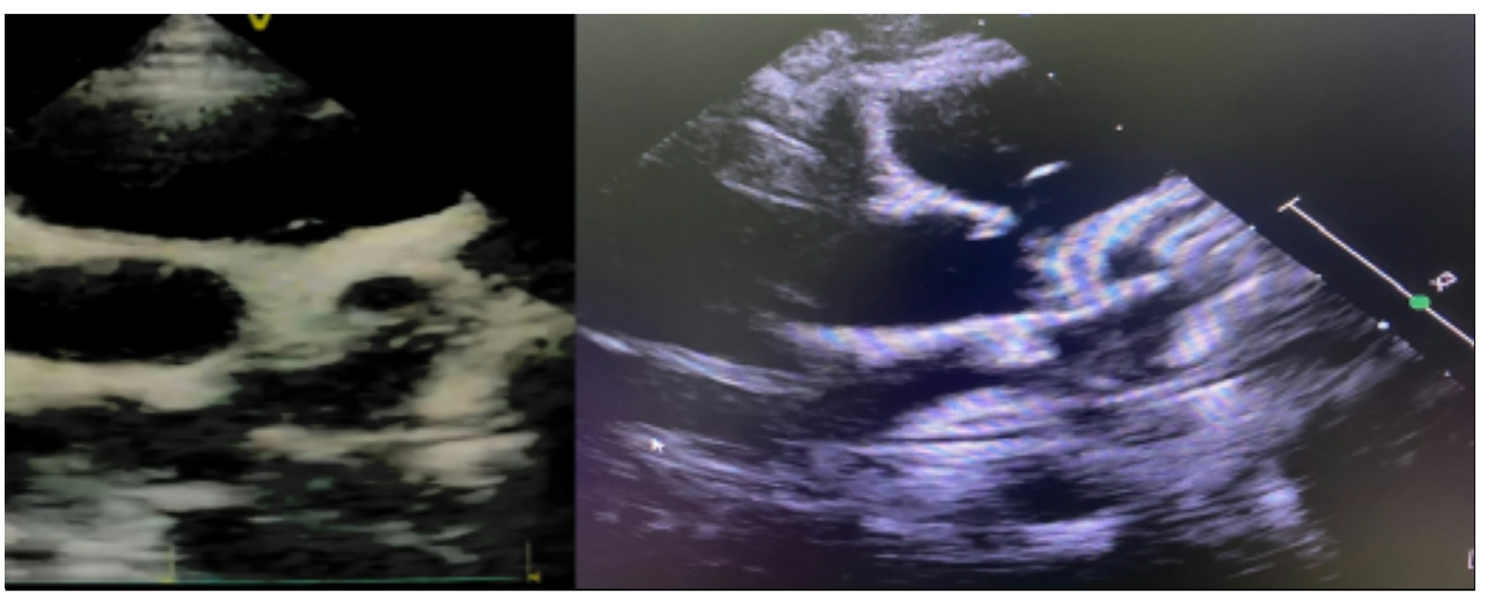
A comprehensive standardized data collection form was used to record patient information, including demographic characteristics, clinical presentations, laboratory findings, and cardiac evaluations. Clinical assessment documented specific manifestations including rash, bilateral non-purulent conjunctivitis, mucocutaneous inflammation (involving oral cavity, hands, or feet), cardiovascular abnormalities (hypotension, shock, cardiac dysfunction, pericarditis, valvulitis, coronary abnormalities), coagulation disorders (prolonged prothrombin time, elevated D-dimer), and gastrointestinal symptoms (diarrhea, vomiting, abdominal pain). Laboratory evaluation included inflammatory markers (erythrocyte sedimentation rate, C-reactive protein, procalcitonin), complete blood count, comprehensive metabolic panel, cardiac biomarkers, and coagulation studies. SARS-CoV-2 exposure was confirmed through PCR testing, serological studies, antigen testing, or documented contact with confirmed COVID-19 cases. Echocardiographic studies were performed by pediatric cardiologists using standardized protocols, with coronary artery dimensions indexed to body surface area and expressed as Z-scores according to established normative data.
Treatment protocol
Management strategies adhered to current clinical guidelines. Standard therapy for KD consisted of intravenous immunoglobulin (IVIG) administration at 2 g/kg as a single infusion, combined with high-dose aspirin (80–100 mg/kg/day). For MIS-C patients, treatment was individualized based on clinical severity and organ involvement, following a predetermined protocol that included immunomodulation and supportive care as needed. Treatment response was monitored through clinical parameters and serial laboratory assessments, with additional therapy initiated for refractory cases according to established algorithms [4].
Follow-up and outcomes assessment
All patients underwent systematic follow-up evaluations at predetermined intervals. These assessments included clinical examination, laboratory testing, and echocardiographic studies. The primary outcomes of interest included resolution of clinical symptoms, normalization of inflammatory markers, and development or resolution of cardiac complications. Coronary artery dimensions were tracked over time and classified according to established z-score criteria.
Statistical analysis
Data analysis was performed using SPSS software version 28 (IBM Corp., Armonk, NY, USA). We expressed continuous variables as mean ± standard deviation or median (range) based on their distribution, as assessed by the Kolmogorov-Smirnov test. Categorical variables were presented as frequencies and percentages. Comparisons between KD and MIS-C groups employed Student’s t-test or Mann-Whitney U test for continuous variables and chi-square or Fisher’s exact test for categorical variables, as appropriate. Statistical significance was set at a two-sided p-value of less than 0.05.