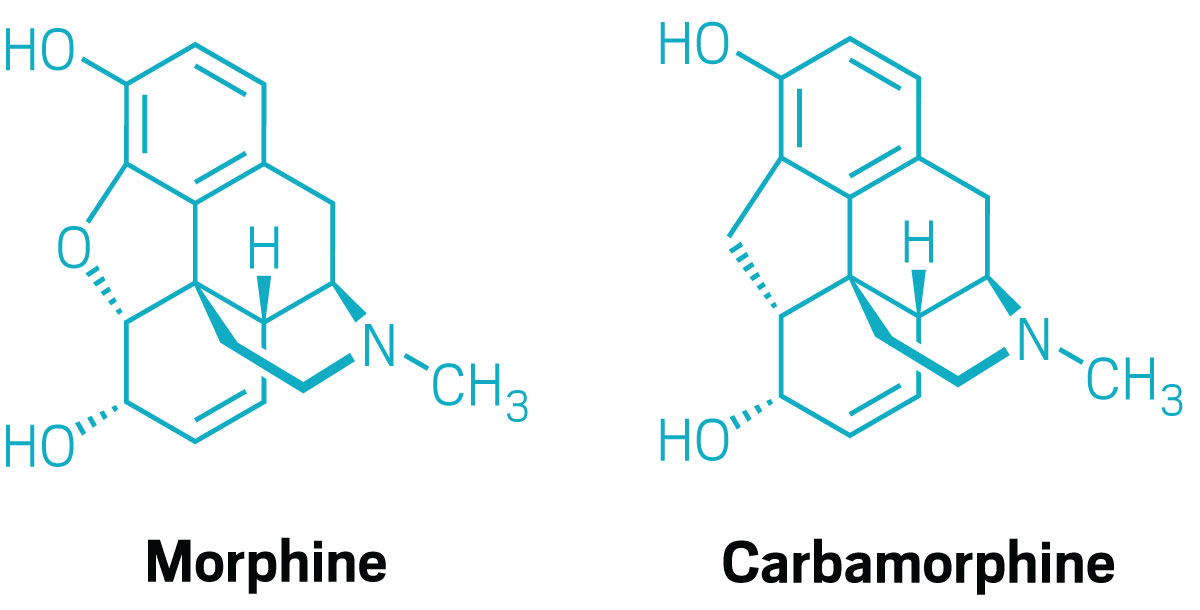
Morphine is one of the most famous natural products in the world and the progenitor of many semisynthetic opioids, such as heroin and oxycodone, as well as the overdose-reversing drug naloxone.
Most of these derivatives were created by making changes to the functional groups on the molecule’s periphery. Researchers led by Richmond Sarpong of the University of California, Berkeley, have now shown that a strategic single-atom change to the molecular core makes a big difference in the compound’s pharmacological activity.
Sarpong and his team synthesized a morphine derivative in which the oxygen atom in one of its central rings has been replaced with a carbon (Proc. Nat. Acad. Sci. U.S.A. 2025, DOI: 10.1073/pnas.2425438122). The new compound, dubbed carbamorphine, offers new insights into designing painkillers with less potential for addiction and overdose—and highlights the power of core-atom changes in medicinal chemistry.
“It’s the first time anybody’s ever made a change to that particular site in morphine,” Sarpong says.
One of the key noncovalent interactions between morphine and the mu opioid receptor protein it activates is a hydrogen bond between the dihydrofuran oxygen and a tyrosine residue in the protein. Switching that oxygen for a carbon was “the most minimal change that we could make to remove the hydrogen bond” while keeping the overall size and shape of the molecule the same, Sarpong says.
Initially, Sarpong had wanted to use a skeletal-editing reaction to directly turn morphine into an atom-swapped analog. That’s still something he hopes to try, but the chemistry needs further development. So he and his team started by building carbamorphine from scratch.
The total synthesis of carbamorphine is a 15-step process and took a year and a half to complete. Sarpong says the most difficult part was creating the molecule’s quaternary carbon center, which the team did using a radical reaction known as the Giese addition. Former postdoctoral researcher Sota Akiyama screened several approaches and ultimately found that a chlorinated intermediate gave the product he wanted in 91% yield.
The chemists used a synthesis route that produced both enantiomers of carbamorphine at the same time, which allowed them to study each mirror-image form’s biological activity. While they were at it, the researchers also made a carbon-swapped version of the opioid antagonist nalorphine.
After Sarpong and coworkers made the new morphine analogs, they teamed up with pharmacologist Susruta Majumdar of Washington University in St. Louis to explore the compounds’ biological activities in vitro and Jay McLaughlin of the University of Florida for mouse studies.
Only one of morphine’s enantiomers activates the mu opioid receptor, but surprisingly, cell studies showed that both enantiomers of carbamorphine activate the receptor. The (–) form of carbamorphine is slightly more potent than the (+) form, but both are less potent than morphine. The researchers chose to focus on (+)-carbamorphine for further testing in mice.
The researchers found that carbamorphine appears to effectively relieve pain in mice without leading to conditioned place preference (a proxy for addiction). It also did not cause breathing problems, even at 10 times the dose of normal morphine that would cause respiratory depression.
“My first reaction was, ‘Wow, how come I didn’t think of this?’ because it totally offers a new way of thinking” about natural products, says Mingji Dai, an organic chemist at Emory University who was not involved in this work but shares Sarpong’s interest in natural products, drug discovery, and skeletal editing. He thinks it’s likely to pique researchers’ interest in exploring precision core-atom changes to bioactive molecules and new methods for making such changes.
That is precisely what Sarpong intended for this research. “It’s really making a forceful case for skeletal editing,” to complement the existing total synthesis approach, he says. And he has no shortage of ideas for ways to follow up on the work, such as swapping other atoms into morphine, further tuning carbamorphine’s properties, and making modifications to naloxone that might extend its half-life. “There’s a lot that’s next” if he can secure funding for it, he says.
Chemical & Engineering News
ISSN 0009-2347
Copyright © 2025 American Chemical Society