A single antibody nasal spray could one day transform allergy relief, as scientists demonstrate powerful protection against pollen-induced sneezing and wheezing in mice.
Study: Intranasal monoclonal antibodies to mugwort pollen reduce allergic inflammation in a mouse model of allergic rhinitis and asthma. Image Credit: Josep Suria / Shutterstock
In a recent study published in the journal Frontiers in Immunology, a group of researchers tested whether intranasal allergen-specific immunoglobulin G1 (IgG1) monoclonal antibody (mAb) prevents mugwort pollen-induced allergic rhinitis and lower-airway inflammation in sensitized mice.
Background
One in three urban residents now reports seasonal sneezing fits as airborne pollen counts climb worldwide, and mugwort blooms are among the chief culprits. Pills and sprays offer momentary relief, while allergen-specific immunotherapy (AIT) demands years of injections before lasting tolerance emerges. Yet emergency rooms still fill when allergic inflammation spills from stuffy noses into wheezing lungs.
Because the nasal mucosa is the first battlefield, scientists are exploring whether an antibody shield sprayed directly onto that surface can defuse pollen before IgE-armed mast cells erupt. Rigorous animal data on such localized passive immunization remain scarce, so further research is urgently needed.
About the study
Researchers produced murine IgG1 mAbs against Artemisia vulgaris (A. vulgaris) pollen by fusing splenocytes from immunized Bagg Albino Laboratory-bred/c (BALB/c) mice with myeloma cells; enzyme-linked immunosorbent assays (ELISAs) identified five clones, and clone XA19 best inhibited human and murine IgE binding to Art v 1. Purified XA19 was given intranasally to male BALB/c mice (20 μg, roughly 1 mg/kg) one hour before each of three allergen challenges.
Sensitization employed two intraperitoneal injections of pollen extract adsorbed to alum, followed by aerosol plus intranasal challenges delivering 1,000 protein nitrogen units (PNU) of extract.
Control groups received phosphate-buffered saline (PBS) alone or pollen without antibody. Outcomes included ear-swelling hypersensitivity, frequency of nasal rubbing, and airway hyperresponsiveness (AHR) measured by whole-body plethysmography after methacholine.
Researchers performed blinded histopathology on lung and nasal tissues. They quantified T helper type 2 (Th2) cytokines, interleukin 4 (IL-4) and IL-5, in tissue homogenates using an ELISA. Serum total and allergen-specific IgE concentrations were also measured. Statistical analysis was used to compare groups, with p < 0.05 considered significant. Group sizes were limited (n=5 per group) in accordance with animal ethics protocols.
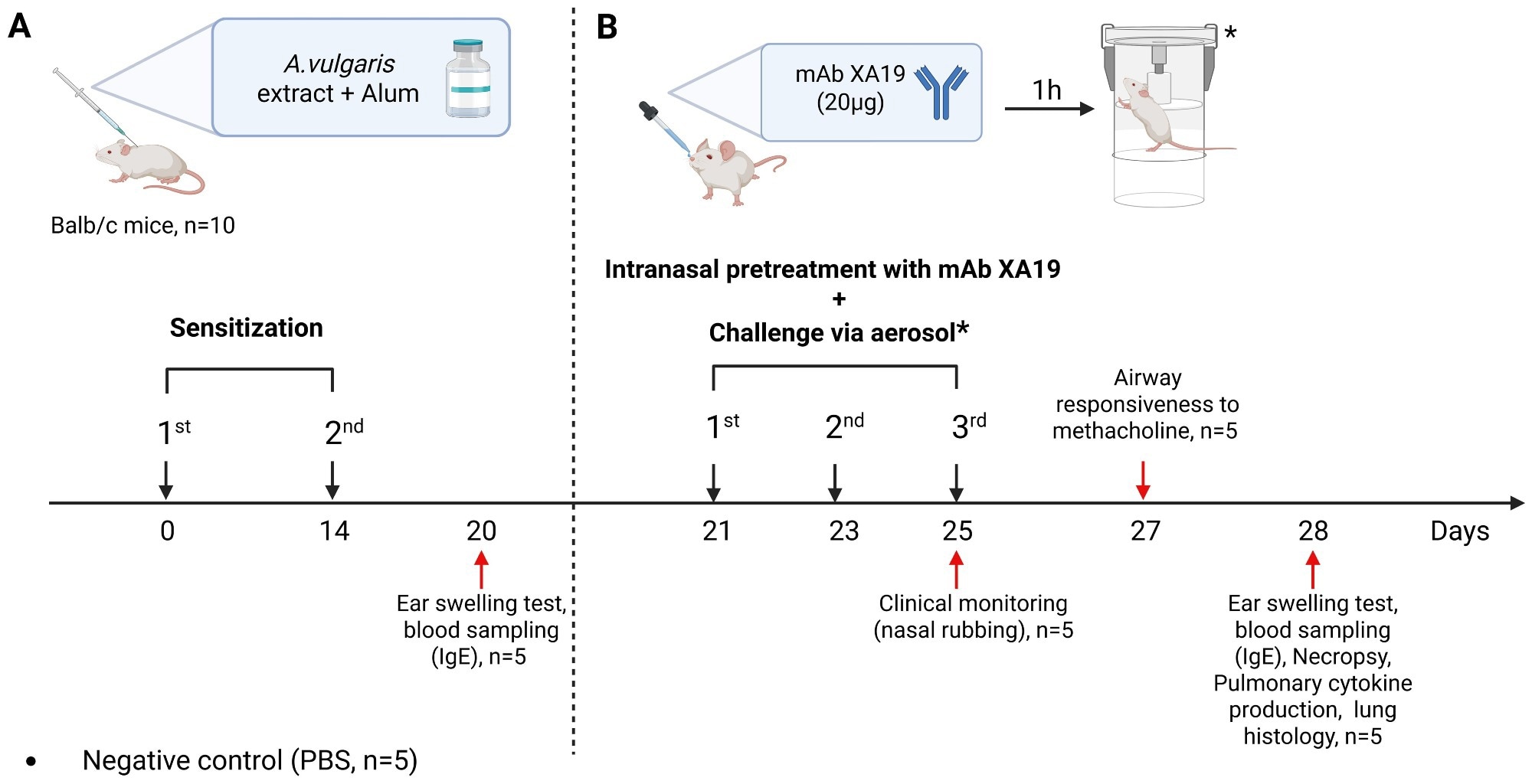 Study design. The schematic illustration shows the sensitization of mice with Artemisia vulgaris pollen extract (A), intranasal pretreatment with the monoclonal antibody XA19 and following subsequent allergen challenge (B).
Study design. The schematic illustration shows the sensitization of mice with Artemisia vulgaris pollen extract (A), intranasal pretreatment with the monoclonal antibody XA19 and following subsequent allergen challenge (B).
Study results
In vitro, XA19 reduced human IgE binding to crude pollen by 18% and to recombinant Art v 1 by 52%, outperforming all other clones. XA19 also inhibited murine IgE binding to crude pollen extract by 22%. In vivo, both antibody-treated and untreated pollen-sensitized groups displayed high total and allergen-specific IgE, confirming sensitization; circulating titers, however, did not differ significantly after treatment.
Despite unchanged systemic IgE, XA19 pretreatment cut immediate ear-swelling responses by roughly half versus sensitized controls and lowered nasal-rubbing bouts to baseline values observed in PBS-exposed mice.
Pulmonary function mirrored these clinical gains. Untreated sensitized mice exhibited a three-fold rise in AHR, whereas XA19-protected animals showed enhanced pause (Penh) values statistically indistinguishable from naïve controls.
Histologically, the lungs of antibody-treated mice retained intact pleura and alveolar septa, with only mild peribronchial lymphocytic cuffs and sporadic goblet-cell metaplasia; low-grade, focal peribronchial lymphocytic infiltrates and mild goblet cell metaplasia were observed in a minority of bronchi. Eosinophil-rich infiltrates and widespread goblet-cell hyperplasia dominated in untreated counterparts.
Nasal turbinates from the antibody group maintained ciliated epithelium with minimal necrosis and negligible debris, while controls displayed desquamation, lamina-propria remodeling, and dense cellular debris masses. Aggregate inflammation scores decreased from 3.4 to 0.6 (scale 0-9) in the upper airway and from 5.8 to 1.2 (scale 0-7) in the lung.
Cytokine profiling supported these morphologic trends. Lung homogenates of control mice harbored high IL-4 and IL-5 levels typical of a Th2 milieu; antibody pretreatment halved both cytokines, indicating dampened Type 2 polarization.
Post-allergen challenge, interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), and IL-17 levels showed non-significant trends to rise in every cohort, yet the changes did not differ significantly among groups. This pattern indicates that the treatment’s advantage stemmed from suppressing the Th2 response rather than promoting a shift toward Th1 or Th17 immunity.
Computational docking illustrated how XA19’s complementarity-determining regions clasp the cysteine-stabilized “defensin-like” head of Art v 1, a domain rich in human IgE epitopes, likely causing steric hindrance.
While robust protection was observed, some mild residual pathological changes remained in treated animals, indicating that the effect was not absolute. However, the precise mechanism by which XA19 blocks the broader allergic response, given mugwort pollen’s multiple allergens, and whether it directly prevents mast cell or basophil activation, remains to be elucidated.
Because mugwort pollen contains various allergens, the partial blockade of extract-reactive IgE still translated into robust protection, underscoring the dominance of Art v 1 in driving disease.
Together, these data show that a single, locally delivered mAb can intercept allergen at the portal of entry, prevent nasal priming, and stop inflammation from cascading into the lower airways without altering systemic IgE production.
Conclusions
To summarize, intranasal administration of the allergen-specific IgG1 mAb clone XA19 provided rapid, non-invasive, and wide-ranging protection against mugwort pollen-induced rhinitis and asthma in mice. By neutralizing allergen at the mucosal front line and lowering IL-4- and IL-5-driven inflammation, the antibody maintained normal breathing and preserved airway architecture even though IgE titers stayed high.
No local irritation or distress was seen in the animals, although formal toxicity was not assessed in this study. The findings are preliminary, based on a small mouse cohort, a single antibody dose, and no isotype control group.
Additional studies are needed to optimize dosing, confirm specificity, and assess long-term safety. These findings highlight localized passive immunization as a promising add-on or alternative to years-long AIT, suggesting that a user-friendly nasal spray could help manage pollen-season symptoms and reduce healthcare burdens once humanized antibodies and muco-adhesive formulations are fully developed.
Journal reference:
- Tabynov K, Nedushenko I, Tailakova E, Sergazina A, Bolatbekov T, Fomin G, Nurpeissov T, Vaghasiya U, Petrovsky N, Demyanov A, Lebedin Y and Tabynov K (2025) Intranasal monoclonal antibodies to mugwort pollen reduce allergic inflammation in a mouse model of allergic rhinitis and asthma. Front. Immunol. 16. DOI: 10.3389/fimmu.2025.1595659, https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2025.1595659/full
