Editor’s Note:
The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians, but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please email us at ccsuggestions@medscape.com with the subject line “Case Challenge Suggestion.” We look forward to hearing from you.
Background
A 42-year-old comatose man is brought to the emergency department (ED) by ambulance. He had recently been hospitalized for decompensated hepatitis C virus liver cirrhosis at another hospital, from which he left against medical advice. In the hours before admission to the ED, the patient experienced two witnessed episodes of loss of consciousness associated with urinary incontinence and myoclonic jerks.
The patient’s prescribed medications include abacavir, lamivudine, and zidovudine daily for HIV infection. He also takes furosemide (50 mg), potassium canrenoate (an aldosterone antagonist), lorazepam, and methadone (90 mg); the latter is for the management of heroin addiction.
Physical Examination and Workup
Upon physical examination, the patient is lethargic, and his Glasgow Coma Scale score is 6 (eye opening response, 1; verbal response, 1; motor response, 4). His pupils are normal in size and bilaterally reactive to light. He has a temperature of 96.8°F (36.5°C), a blood pressure of 90/54 mm Hg, and a pulse rate of 86 beats/min. His respiratory rate is 18 breaths/min, and he has an oxygen saturation of 98% while breathing room air.
Upon auscultation, the lung fields are clear bilaterally, and normal heart sounds are heard. His peripheral pulses are palpable; however, bilateral lower extremity pitting edema is present. The abdomen is distended, tense, and with ascites. His sclerae are noted to be icteric.
Laboratory tests are ordered, with pertinent findings that include a hemoglobin level of 11.1 g/dL (reference range, 13.5-17.5 g/dL) and a platelet count of 24 × 109/L (reference range, 136-436 × 109/L).
A chemistry panel reveals the following:
- Sodium level: 134 mEq/L (134 mmol/L; reference range, 135-145 mEq/L)
- Potassium level: 3.2 mEq/L (3.2 mmol/L; reference range, 3.5-5 mEq/L)
- Creatinine level: 0.6 mg/dL (53.04 µmol/L; reference range, 0.7-1.2 mg/dL)
- Glucose level: 148 mg/dL (8.21 mmol/L; reference range, <140 mg/dL)
- Bilirubin level: 4.7 mg/dL (80.37 µmol/L; reference range, 0.3-1 mg/dL)
- Magnesium level: 1.3 mg/dL (0.53 mmol/L; reference range, 1.5-2.5 mg/dL)
- Ammonium level: 153.3 µg/dL (90 μmol/L; reference range, 11-79 µg/dL)
- Ionized calcium level: 3.96 mg/dL (0.99 mmol/L; reference range 4.6-5.6 mg/dL)
His troponin level is 0.07 ng/mL (0.07 μg/L; reference range, <0.12 ng/mL). Serum alcohol testing results are negative, and a urine toxicology screen is negative for cannabinoids, cocaine, and opiates (note that methadone usage may not cause a positive opiate result). A CT scan of the brain is negative for acute abnormalities.
The patient is initially thought to have had a seizure and is cautiously given benzodiazepines to prevent a recurrence.
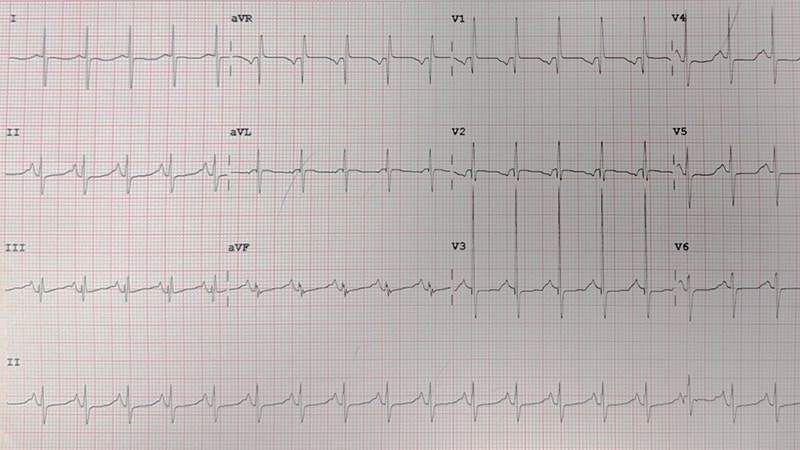
An electrocardiogram (ECG) is then performed (Figure 1). Soon afterwards, an abnormal tracing is seen on the cardiac monitor (Figure 2), and the patient becomes pulseless and apneic and requires cardiopulmonary resuscitation.
Figure 1.
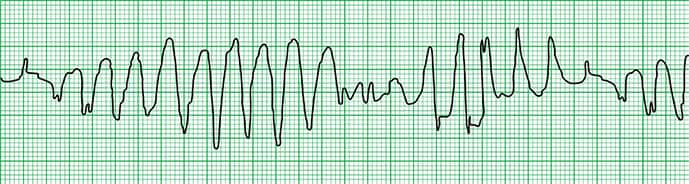
Figure 2.
Discussion
The cardiac rhythm strip (Figure 2) demonstrated torsade de pointes (French for “twisting of the points”), otherwise known as simply “torsades” or polymorphic ventricular tachycardia.

Figure 2.
The initial ECG (Figure 1), which was obtained before the development of the torsades, revealed a prolonged QT interval and notched T waves. A prolonged QT interval is often noted incidentally on an ECG in an asymptomatic patient; however, in a patient who presents with palpitations, presyncope, syncope, or cardiac arrest, the presence of a prolonged QT interval should raise particular concern for torsade de pointes.
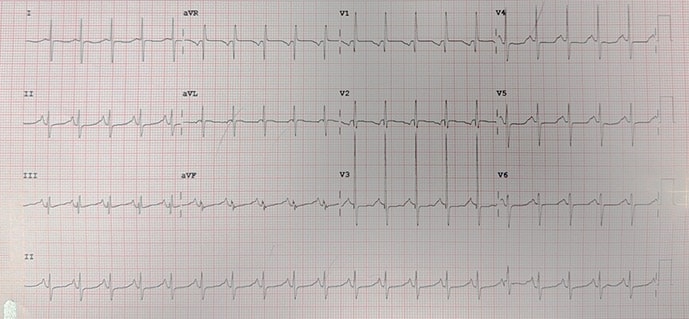
Figure 1.
QT prolongation can be either acquired or congenital. A thorough clinical history-taking and knowledge of the patient’s current medications is very important for this differentiation. Congenital long QT syndrome (LQTS) is a disorder characterized by abnormal QT-interval prolongation on the ECG caused by cardiac myocyte ion channel gene mutations, with a propensity to ventricular tachyarrhythmias. Patients are typically young and may present with syncope or sudden death.[1,2,3,4]
Acquired QT interval prolongation may be drug-induced, or it may be caused by certain electrolyte derangements, such as hypomagnesemia, hypokalemia, and hypocalcemia. Many drugs have been implicated, including class 1A antiarrhythmic drugs such as quinidine and procainamide and class III antiarrhythmics such as amiodarone and sotalol. Other drugs that have been implicated include antihistamines (terfenadine, astemizole), macrolide antibiotics (erythromycin, clarithromycin, clindamycin, azithromycin), pentamidine, serotonin receptor antagonists (ketanserin), diuretics (indapamide), certain fluoroquinolone antibiotics, tricyclic antidepressants, antipsychotics (phenothiazines, haloperidol, mesoridazine, pimozide, thioridazine, ziprasidone), gastrointestinal motility enhancers (cisapride, domperidone), inotropes (amrinone, milrinone), antimalarials (chloroquine, hydroxychloroquine), toxins (organophosphates, arsenic), protease inhibitors, and methadone.[1,5,6,7,8,9]
Drug-induced prolongation of the QT interval is directly linked to a modification in myocardial cell repolarization, which is mediated by the efflux of potassium ions. The shape of the action potential depends on the balance between sodium and calcium inflow and potassium outflow. Two subtypes of the delayed rectifier K+ current, IKr (rapid) and IKs (slow), are responsible for repolarization. The human ether-a-go-go related gene (hERG; also termed KCNH2) codes for a protein known as the Kv 11.1 potassium ion channel, which mediates the repolarizing potassium current IKr.
Blockage of the hERG-encoded potassium channels has been implicated as a cause of drug-induced QT prolongation. A strong correlation is noted between IKr blockade and ventricular arrhythmia or sudden death. Drugs that block the IKr channel increase the QT interval and allow inward current, particularly calcium, to reactivate, leading to early after-depolarizations in cardiac tissue that may result in torsades. Other drugs implicated in QT prolongation have no effect on the potassium channels; therefore, additional cardiac mechanisms can play a significant role.[2,5]
Some medications prolong the QT interval at specific doses, whereas others may act at any dose. The patient’s underlying decompensated hepatitis C virus liver cirrhosis is a significant predisposing factor for QT interval prolongation and the development of torsades.[4] Many medications that prolong the QT interval, including methadone, are metabolized by the hepatic cytochrome P450 3A4 (CYP3A4) system. Therefore, hepatic dysfunction can alter drug metabolism and increase plasma concentrations of QT-prolonging drugs, thereby contributing to QT prolongation. This risk is further exacerbated by potential drug-drug interactions involving CYP3A4 inhibitors.
When administering a drug that potentially prolongs the QT interval, numerous predisposing factors for torsades development must be considered, including advanced age, obesity, poor nutrition (anorexia nervosa, starvation diets, alcoholism), bradycardia (<50 beats/min), cerebrovascular disease (intracranial and subarachnoid hemorrhage, stroke, intracranial trauma, thalamic hematoma), congenital long QT syndrome, heart failure (cardiomyopathy, dilated or hypertrophic), hypoglycemia, hypothermia, hypothyroidism, myocardial ischemia or infarction, organophosphate exposure, pheochromocytoma, pituitary insufficiency, coadministration of other QT prolonging agents, and hypoxia.
If a drug-to-drug interaction is suspected, the drug should be withdrawn. Ehret and colleagues[9] studied a population of active or former intravenous drug abusers and suggested that methadone (even at low doses), CYP3A4 inhibitors, and hepatic dysfunction contributed to prolongation of QT. Methadone delays cardiac repolarization by blocking the flow of potassium ions through the hERG channels, but no evidence suggests interaction between this drug and nucleoside reverse transcriptase inhibitors.[5,7,9,10]
Torsade de pointes is characterized by QRS complexes that vary in axis and amplitude over the isoelectric line (“twisting around the points,” as the name implies). Other associated characteristics include the presence of long and short beat-to-beat (RR) interval onset after an early premature ventricular contraction.
A relationship between the degree of QT interval prolongation and the development of torsades is noted. The QT interval varies directly with heart rate, and a correction is required in order to compensate for heart rate. A commonly used correction (QTc) is the Bazett correction (QTc=QT/√RR), wherein QT is the longest QT interval measured on the ECG, and RR is an average RR interval. QT measurement should be made manually from a 12-lead ECG, and it is calculated from the beginning of the QRS complex to the end of the T wave and averaged over three to five beats in a single lead. Prominent U waves should be included in the measurement if they merge into the T wave. It is advisable to assess QT during peak plasma concentration of any ingested QT-prolonging substances and to correct it for heart rate while looking for other warning signs, including the appearance of prominent U waves, extrasystoles, and U wave augmentation after extrasystole.
Corrected QT is considered prolonged if it is beyond 440 msec for adult males, 460 msec for adult females, and 500 msec in the presence of ventricular depolarization abnormalities (ie, bundle branch blocks or intraventricular conduction delay greater than 120 msec). The uncorrected QT interval should also be considered, however, as a very long QT (>600 msec) after drug exposure is a marker of an increased risk for torsades.[1,5,11]
The patient in this case was initially treated with 2 g of intravenous magnesium sulfate and 1 g of calcium chloride. Despite this, he developed recurrent torsade de pointes. He underwent repeated defibrillation followed by irregular rhythms, including premature atrial complexes and ventricular bigeminy. The recurrent episodes of torsade de pointes were then treated with an intravenous bolus of lidocaine followed by a 2-mg/min infusion. Normal sinus rhythm then returned, and the patient slowly improved and regained consciousness.
In this case, the cause of the patient’s QT prolongation was likely multifactorial and probably included the chronic use of methadone and electrolyte derangement. Slight hypomagnesemia, hypocalcemia, and hypokalemia were noted. These mild electrolyte abnormalities alone are not sufficient to result in torsade de pointes, as evidenced by the persistence of episodic torsade de pointes despite electrolyte replacement. Once the methadone was withdrawn, however, no further episodes of torsade de pointes occurred, and the QT interval normalized.
Cirrhotic cardiomyopathy is often associated with QT interval prolongation and is an important consideration in the differential diagnosis of acquired QTc prolongation.[12,13] However, in this patient’s case, the arrhythmogenic effect was more likely medication-related than a direct consequence of cirrhosis, given the resolution of QTc prolongation following methadone withdrawal.
Treatment of Torsade de Pointes
The immediate and primary treatment for torsades is intravenous magnesium sulfate, typically as a 2-g bolus followed by an infusion of 2-4 mg/min. This therapy is recommended regardless of the patient’s serum magnesium levels.[3] Repeated doses may be needed, titrated to suppress ectopy and nonsustained ventricular tachycardia episodes while precipitating factors are corrected.[3] Magnesium is not contraindicated in liver disease, although caution may be necessary in severe renal failure.
For patients experiencing hemodynamically unstable torsades, or if the arrhythmia persists or degenerates into ventricular fibrillation (VF), immediate unsynchronized defibrillation (direct current cardioversion) is indicated.[3,4]
Correction of electrolyte abnormalities is crucial. Serum potassium levels should be maintained in the high-normal range (4.5-5 mmol/L).[3,4] Hypokalemia and hypomagnesemia are recognized factors that can trigger ventricular arrhythmias, including torsades. If both magnesium and potassium levels are low, magnesium should be repleted first to facilitate potassium replacement.
For recurrent torsades, particularly in patients with acquired long QT syndrome and bradycardia that is refractory to magnesium, rate-increasing therapies are recommended to shorten the QT interval and prevent recurrences. These therapies include the following:
- Overdrive transvenous pacing: Pacing with a slightly higher rate than the baseline rhythm can temporarily suppress slow recurrent or incessant VT.
- Isoproterenol infusion: Isoproterenol works by increasing heart rate and abolishing the post-extrasystolic pauses that can precipitate torsades. However, it is contraindicated in patients with congenital long QT syndrome and ischemic heart disease.[3,4] Maintaining a heart rate greater than 70 beats/min protects against drug-induced torsades.[2,3,4]
Any offending QT-prolonging medications should be discontinued whenever drug-induced arrhythmias are suspected. Medications such as amiodarone and procainamide, which prolong the QT interval, should not be used for torsades or arrhythmic storm associated with a prolonged QT, as they can worsen the condition.[2,3] Patients who require a potentially arrhythmia-inducing drug should undergo regular ECG and other tests based on their profile and the drug’s characteristics.[4]
While not a first-line approach to acutely terminate torsades, beta-blockade is a component of the multifaceted approach to managing arrhythmic storm (which can include torsades).[2] Nonselective beta-blockers (eg, propranolol) are preferred and often used in combination with intravenous amiodarone in patients with structural heart disease (SHD) and electrical storm, unless contraindicated.[2,3,4]
Lidocaine, a class IB antiarrhythmic, blocks fast inward sodium currents and can slightly shorten the QTc interval. It is included in some guidelines for torsades and electrical storm management and is considered beneficial for 4 cardiac arrest due to shock-refractory VF or polymorphic VT.[3,4] It may be considered if beta-blockers and amiodarone are ineffective.[4]
Long-term management and prevention of sudden cardiac death in patients susceptible to torsades and other ventricular arrhythmias primarily involves implantable cardioverter-defibrillators (ICDs) and comprehensive medical strategies.[2,3,4]
This case illustrates an incident of likely drug-induced torsade de pointes resulting from methadone usage in the setting of electrolyte abnormalities. The case highlights the need for an evaluation for potential cardiogenic causes of syncope in patients who present with an abnormal ECG.
Acquired QT interval prolongation may be drug-induced, or it may be caused by certain electrolyte derangements, such as hypomagnesemia, hypokalemia, and hypocalcemia. Many drugs have been implicated, including class 1A antiarrhythmic drugs such as quinidine and procainamide and class III antiarrhythmics such as amiodarone and sotalol. Other drugs that have been implicated include antihistamines (terfenadine, astemizole), macrolide antibiotics (erythromycin, clarithromycin, azithromycin, clindamycin), pentamidine, serotonin receptor antagonists (ketanserin), diuretics (indapamide), certain fluoroquinolone antibiotics, tricyclic antidepressants, antipsychotics (phenothiazines, haloperidol, mesoridazine, pimozide, thioridazine, ziprasidone), gastrointestinal motility enhancers (cisapride, domperidone), inotropes (amrinone, milrinone), antimalarials (chloroquine, hydroxychloroquine), toxins (organophosphates, arsenic), protease inhibitors, and methadone.
Immediate treatment for patients who develop torsades can be categorized into pharmacologic and nonpharmacologic approaches. Intravenous magnesium sulfate (2-g bolus followed by an infusion of 2-4 mg/min) is the initial therapy, regardless of the serum levels of magnesium.
Editor’s Note: This article was created using several editorial tools, including generative AI models, as part of the process. Human review and editing of this content were performed prior to publication.