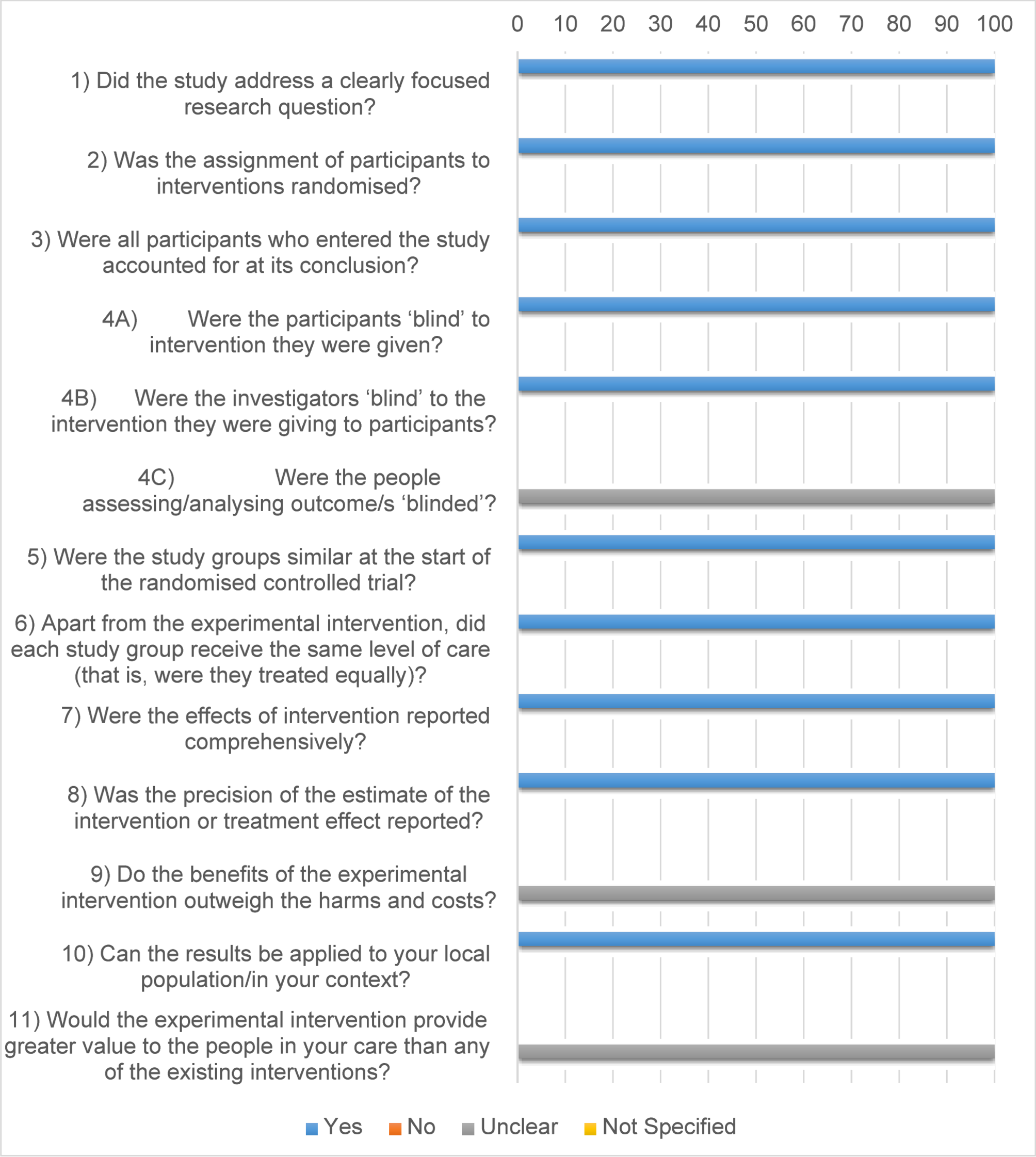
This systematic review examines the relationship between ceftriaxone therapy and cholelithiasis in pediatric patients, synthesizing findings from 11 studies conducted in different countries, including Japan, Switzerland, Turkey, the USA, and…
Ceftriaxone-induced cholelithiasis in pediatrics: pooled frequency, symptoms, and associated factors — systematic review and meta-analysis | Italian Journal of Pediatrics