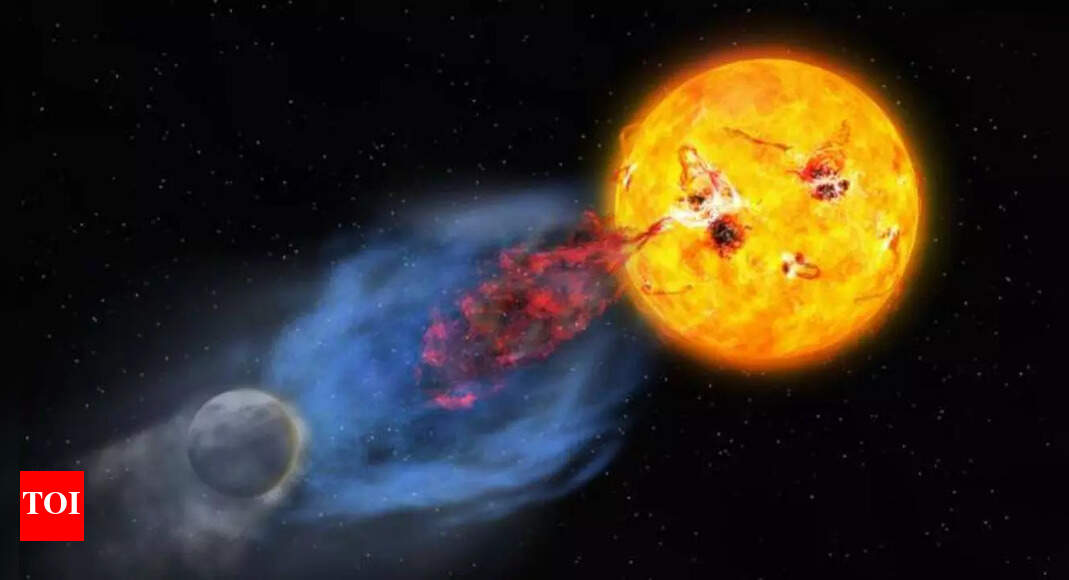
If you think your morning mood swings are bad, wait until you hear what young stars do. Instead of coffee, they start their day by hurling billions of tonnes of plasma into space, a sort of cosmic temper tantrum. Scientists say our own Sun…
Hubble and ground telescopes capture the first multi-temperature Plasma eruption from a young sun-like star |