Textbooks often depict proteins in one conformation, but real life, as usual, is much messier. While some proteins have stable, unchanging structures, many others have intrinsically disordered regions, or areas where the structure is unstable and thus subject to change. Some entire proteins are disordered as well, not just certain regions. These protean proteins make up a large swath of the human proteome—a preprint in 2024, which was not peer-reviewed, showed that almost 60% of the human proteome had at least one intrinsically disordered region.
If one wants to design a small molecule to target a stable protein, the task is usually to find a pocket in the structure where the molecule can land. But drugging a protein that has disordered regions is much more difficult because the target is either moving or nonexistent.
These proteins can have too many conformations for researchers to keep track of, says Kejia Wu, a postdoctoral researcher working with David Baker at the Institute for Protein Design at the University of Washington. “We don’t have reliable experimental methods to characterize all their possible conformations.”
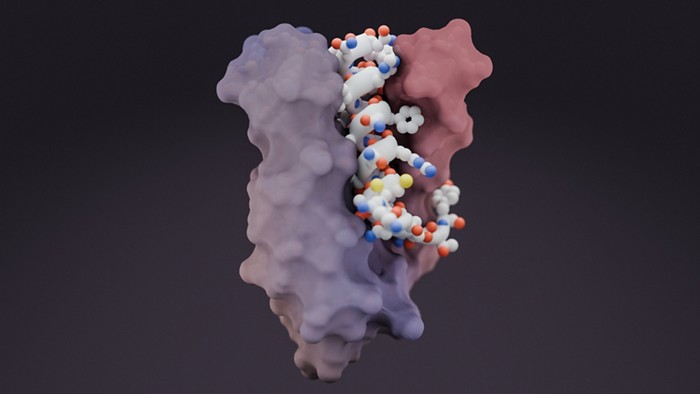
Now, with two artificial intelligence–based systems, researchers can target these disordered proteins. In a preprint on bioRxiv, published without peer review, Wu and colleagues describe a deep learning–based approach with the Baker lab’s RFdiffusion program. The researchers feed the program an amino acid sequence that the team wants to target—not the—and the program designs a binder that’s also flexible. When brought together, this flexible binder and the target protein do a bit of a dance as they find a stable way to bind together. This approach has the benefit of not trying to pin down a constantly changing conformation because the amino acid sequence stays the same, regardless of the shape the protein makes.
This method “works well for cases where the disordered protein can adopt some helix or strand conformation or a mixture of both,” Baker says. For example, the binder and target can adopt similar conformations to those found between proteins that are not intrinsically disordered.
A recently published paper in Science from the same group builds on this idea with a technique called “logos” that can step in when RFdiffusion may struggle (2025, DOI: 10.1126/science.adr8063). Logos takes a somewhat contrary approach: instead of finding a small molecule to fit in the pocket of an ever-changing protein, the binders this technique creates are their own pockets that the target fits into. This technique, Baker says, “works best for cases where that sequence that you’re trying to target really doesn’t particularly want to be in a strand or helix.” In those situations, “modeling and treating it as an extended chain will always work.”
Since these disordered proteins are so common, being able to target them opens up the ability to target many more proteins than previously possible, like proteins involved in cancer and genetic regulation. Wu highlights neurodegenerative diseases as one area where these enveloping binders could shine.
“Humans are living longer in modern society and suffering more from neurodegenerative disease,” she says. For example, the intrinsically disordered protein tau has been implicated in Alzheimer’s disease, and these binders could disrupt its damaging aggregation.