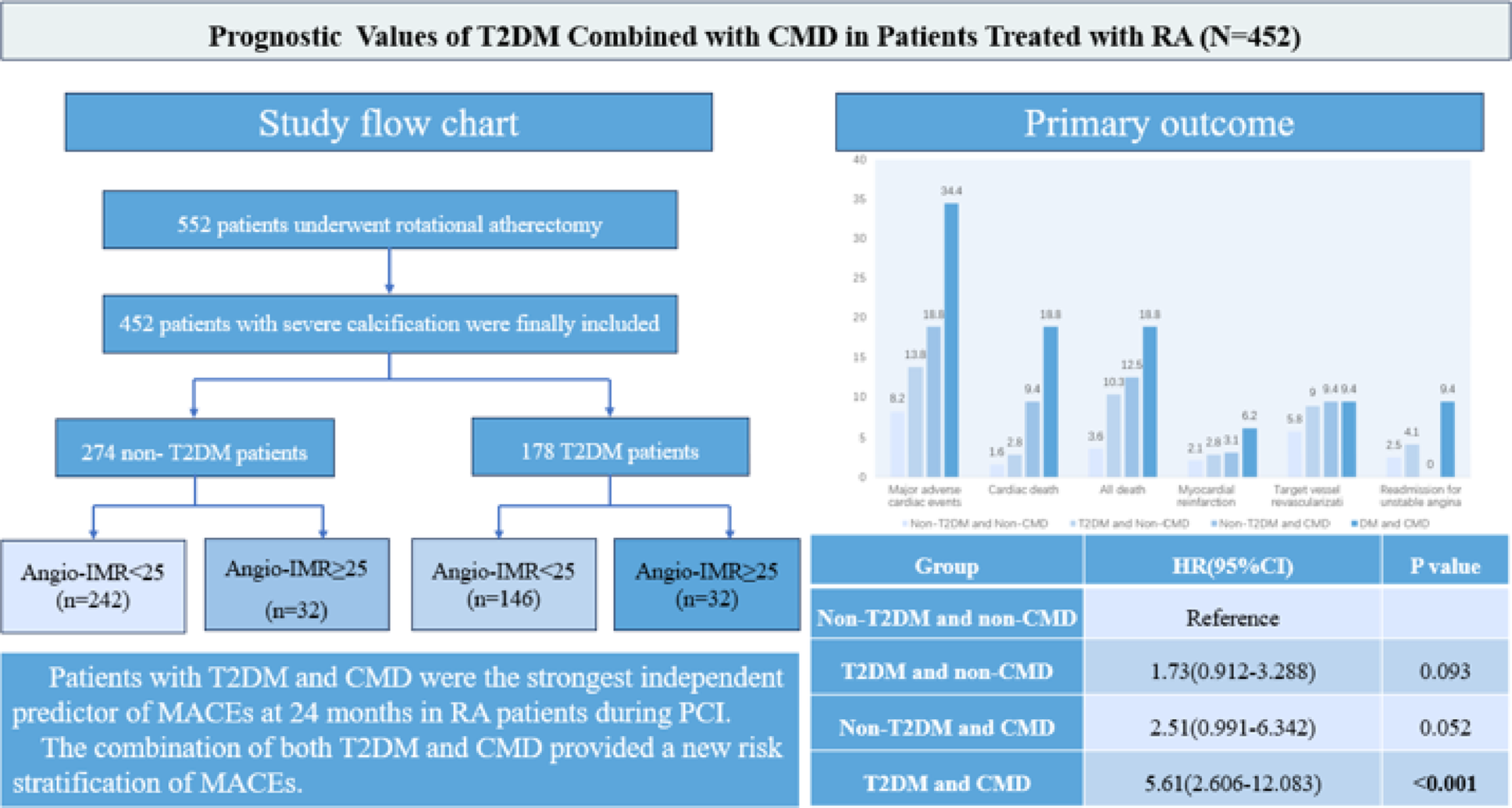
The first study demonstrated combined prognostic significance of T2DM and by angio-IMR- assessed CMD in severe coronary artery calcification treated with RA during the PCI. The primary findings of the study were as follows: (1) RA exacerbated microvascular dysfunction in T2DM patients. (2) T2DM patients exhibited a heightened MACE risk than non-DM patients. (3) Vessels with CMD had a higher risk of MACEs relative to those without CMD. (4) Patients with both T2DM and CMD exhibited an elevated combined risk of MACEs. (5) T2DM and CMD were independent risk factors of MACEs. These results highlight the importance of evaluating CMD in patients undergoing RA and provide new risk stratification insights for diabetic.
DM was significantly associated with coronary artery calcifications and CMD [3, 39]. DM has been proven to promote the proliferation and migration of vascular smooth muscle cells and osteogenic trans-differentiation, thereby accelerating coronary artery calcification progression. It has been shown that DM adversely affects endothelial cell function and exacerbates endothelial dysfunction [5, 14]. The increased production of advanced glycation end-products (AGEs) has been demonstrated to result in oxidative stress and inflammatory responses. Hyperglycemia has been linked to increased blood viscosity, while abnormal lipid metabolism results in lipid deposition. The microvascular basement membrane undergoes a process of thickening, attenuation of microvascular vasorelaxation, narrowing of the lumen, and enhanced endothelial thrombogenicity. Collectively, these factors impact blood flow and contribute to the development of coronary microcirculation disorders [5, 40]. Furthermore, the vibration generated by rotational atherectomy had been demonstrated to exert an impact on the hyperemia of the coronary arteries [41], thereby likely impacting the coronary microvascular.
The presence of coronary calcification predicted an elevated risk of 2-year MACEs and bleeding, both before and after adjustment for confounding factors [42]. In addition, coronary calcified plaque length was associated with stent under expansion, and effective calcium fracture is critical to improving outcomes in severely calcified lesions, so calcified plaque modification prior to stent expansion is very important [11, 43]. For the modification of calcified coronary plaques, RA and intravascular lithotripsy were both effective techniques [23]. Nonetheless, RA remains the dominant atherectomy modality for inpatient PCI in the US (93%) [12] and in our centers. One study found that the number of burrs used in RA had significant association with the prognosis of RA patients [44]. It was not deemed an independent risk factor in this study. Moreover, Plaques were modified by RA into particles that induced significant angio-IMR elevation from pre- to post-PCI. RA in PCI for coronary calcification could increase risk of microvascular injury. Microcirculation protection needed to be enhanced during rotational atherectomy.
The CMD encompasses both obstructive and non-obstructive coronary arteries disease. Obstructed coronary arteries could reduce perfusion pressure and lead to structural and functional changes in the distal microvasculature [45].The present study investigated the coronary arteries obstruction in patients with DM. El Farissi et al. [46] reported 5-years cardiac mortality rates of 2.2% and 4.9% in patients withIMR ≤ 40 and IMR > 40, respectively, and IMR was an independent predictor of cardiac mortality. Patients with IMR < 25 exhibited a lower risk of MACEs at 12 months compared to those with 25 ≤ IMR ≤ 40 and IMR > 40 [47]. The present study revealed that patients with CMD had a significantly higher risk of MACE than patients without CMD (26.8% vs. 10.9%, p < 0.001), similar to the previous studies.
It has been demonstrated that DM resulted in microvascular impairment, thereby contributing to the development of CMD [5]. The findings of the present study indicated that DM was associated with an increased risk of MACEs at the 24-month follow-up, with a statistically significant difference observed between patients with DM and those without DM (18.2% vs. 9.6%, p = 0.011). This difference became more significant as the follow-up time increased. In addition, the present study indicated that DM following RA was associated with exacerbated microvascular dysfunction. The previous study found that CMD was significantly elevated in patients with DM compared to those without DM (57.8% vs. 38.3%, p = 0.001) [48]. In study of stable CAD, the high IMR (IMR ≥ 25; HR, 1.57(1.15–2.14), P = 0.005) and DM were independently associated with a higher risk of MACEs [49]. These studies were similar to our study that patients with both DM and CMD had an increased risk of MACEs. The patients in this study were divided into four groups according to DM and CMD, and the observed difference in MACEs among the four groups was mainly attributable to variation in cardiac mortality. These patients presented with severely calcified lesions, and the majority exhibited multivessel disease, resulting in poor long-term outcomes. Multivessel disease is more likely to impair the blood flow, leading to circulatory collapse and an increased risk of cardiac death. IMR was an independent predictor of cardiac mortality [46]. DM and CMD each independently increased cardiac mortality risk, with their co-occurrence demonstrating synergistic effects. These may be the reasons why the difference in MACE was driven by cardiac death. Patients treated with RA and concomitantly with both DM and CMD exhibited an elevated risk of MACEs. In summary, the combination of both DM and CMD require intensified risk stratification of MACEs in RA patients during PCI.
DM contributes to coronary microvascular dysfunction and accelerates coronary calcification. Notably, calcified plaques modify by RA may generate microparticles or microthrombi, which are subsequently absorbed by the distal reticuloendothelial system, thereby inducing distal microvascular injury. In this study, RA-modified calcification significantly affected angio-IMR levels (13.22 [10.32–17.61] vs. 18.04 [14.10-21.98]). Elevated post-RA angio-IMR (≧ 25) further correlated with adverse clinical outcomes, suggesting that the combination of DM and CMD enhances the predictive accuracy of risk models for MACEs. Drawing on these findings, we recommend routine angio-IMR assessment of coronary microcirculation in every patient undergoing RA during PCI. This is particularly imperative for individuals with T2DM, in whom post-PCI angio-IMR should be standard measurement. For those who have both T2DM and confirmed post-PCI CMD, immediate in-hospital intensification of glycemic control and initiation of endothelial-protective therapy are advised, followed by structured outpatient follow-up. This surveillance includes semiannual microcirculatory assessment and modulation of cardiovascular risk factors. Triple therapy (statins, ACE inhibitors, and calcium channel blockers) is recommended for patients exhibiting both endothelial dysfunction and atherosclerosis [50]. Furthermore, nicorandil acting via dual nitrate and K-ATP channel opening mechanisms, has demonstrated efficacy in reducing angina episodes by dilating microvascular, restoring endothelial function post-RA, and improving myocardial perfusion [51,52,53]. There effects may contribute to the amelioration of CMD. Additionally, combining statins with angiotensin-converting enzyme inhibitors is recommended for DM patients with endothelial dysfunction. This combination provides microcirculatory protection post-PCI and improves vascular health synergistically [54], though the optimal treatment duration requires validation through randomized controlled trials. These interventions address the pathophysiological mechanisms linking DM, CMD, and calcification, and potentially optimizing long-term prognosis.
In addition, the aforementioned studies using of pressure wires to quantify the IMR, a methodology that was associated with additional costs, prolonged procedure times, and the administration of other vasodilators, such as ATP or adenosine. However, the present study was used angio-IMR, a novel approach that exhibited a high correlation and diagnostic accuracy for IMR to assess CMD, rather than the pressure wire. The diagnostic performance of angio-IMR in the context of chronic and acute coronary syndrome had been demonstrated to be highly effective [25, 47, 48]. Angio-IMR had been shown to significantly improve the ability to reclassify patients and estimated the risk of MACEs in patients with non-ST-elevation and ST-elevation myocardial infarction [20, 55]. Angio-IMR would be more widely used in coronary artery disease due to superior estimation of clinical outcomes. This facilitates the accurate risk stratification of patients using RA and enhances clinical accessibility, thereby benefiting a greater number of patients (thus enabling expanded patient access).
Limitation
There are several limitations in our study. First, it was a retrospective study, which inevitably introduces selection bias, information bias and follow-up bias. However, our study is a large multicenter study with 24 months of follow-up and all patients met the inclusion criteria and were enrolled consecutively. Second, vasodilators use in RA patients during PCI may influence post-PCI angio-IMR results and has not been collected and analyzed. However, the use of different types of vasodilators was not associated with CMD [18].Third, while RA speed down was an important variable in the context of RA, its collection and analysis had not been undertaken in this study. However, alternative parameters such as burr size, rotational atherectomy speed and rotational atherectomy time may offer some guidance, as evidenced by several studies related to RA [13, 56, 57]. Fourth, the collection of follow-up symptoms and hemodynamic data during PCI was not included in the study. These variables require future collection and analysis. Finally, subgroup analyses demonstrated consistent results between groups, with robustness confirmed by post-hoc sensitivity testing. It should be interpreted with caution, given the exploratory nature of subgroup analyses. Therefore, extrapolation of the clinical implications of our findings should be done with caution and not suitable for patients with type 1 DM.