Plasmids, cells, and viruses
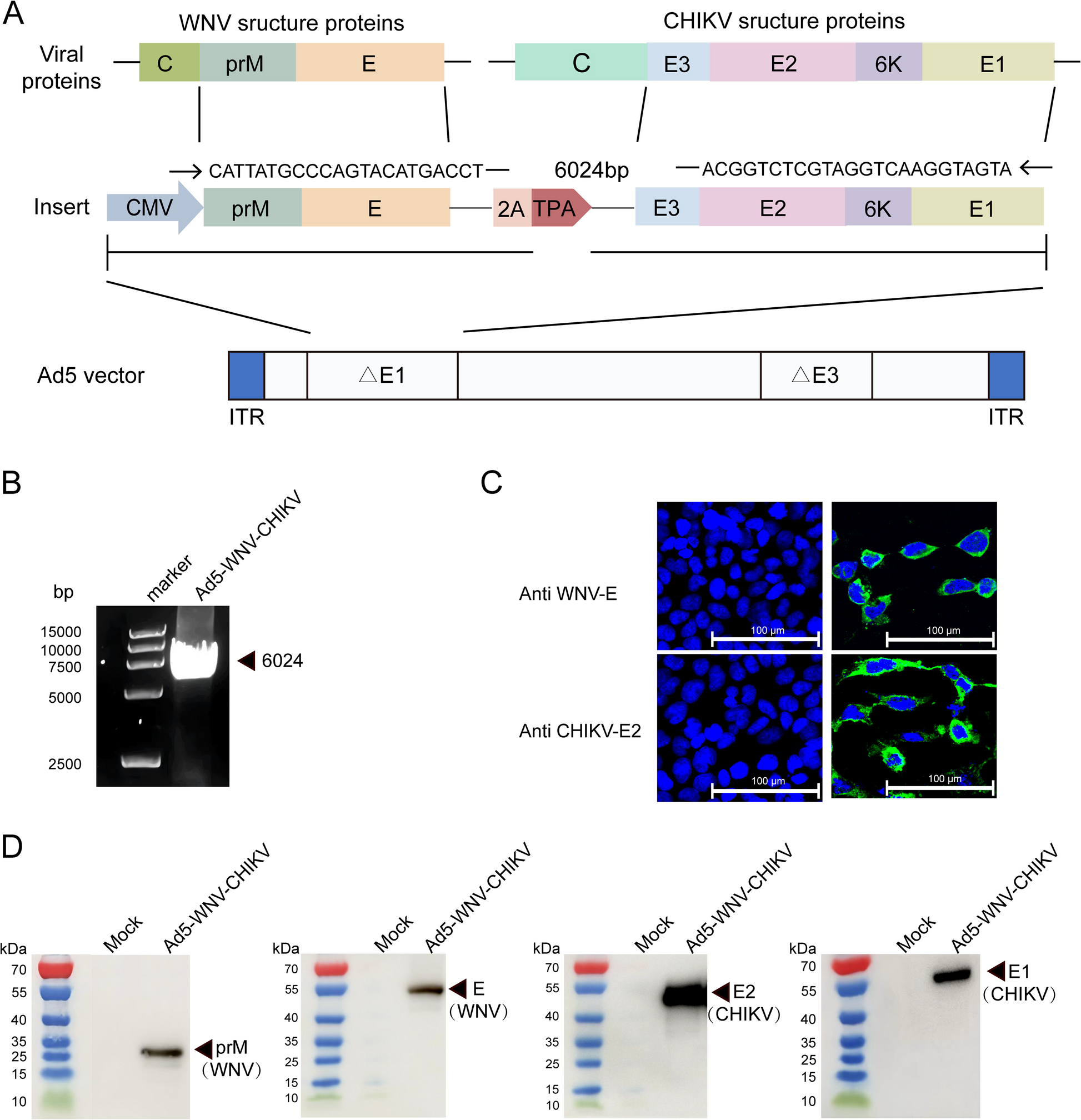
The prM-E coding gene of WNV (WNV-XJ11129-3 strain, GenBank: JX442279) and E3-E2-6 K-E1 coding gene from CHIKV (CHIKV 181/clone25) were linked in tandem using a 2 A peptide. This construct was synthesized through codon optimization and was, subsequently, cloned into the pVRC vector, resulting in a plasmid designated pVRC-WNV-CHIKV (GenScript Co., Nanjing, China). Human embryonic kidney cell lines, including HEK293 (293 cells), HEK293T (293 T cells), and African green monkey kidney cells (Vero cells) were procured from the American Type Culture Collection and stored in our laboratory. Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM; Gibco, Billings, MT, USA) supplemented with 10% fetal bovine serum (Vazyme, Nanjing, China), 1% L-glutamine, and 1% penicillin–streptomycin, and incubated in a humidified chamber with 5% CO2 at 37 °C. The live attenuated CHIKV 181/clone25 virus was provided by Professor Zhang (Shandong First Medical University & Shandong Academy of Medical Sciences, Shandong, China).
Preparation of recombinant adenovirus Ad5-WNV-CHIKV
The target genes prM-E from WNV and E3-E2-6 K-E1 from CHIKV were digested with the restriction enzymes BamHI and EcoRV and subsequently assembled into the PKAd5CMV-NSrfh vector in accordance with established protocols, resulting in the plasmid pKAd5C-WNV-CHIKV. This plasmid was linearized using PacI and then transfected into 293 cells to rescue the recombinant Ad5-WNV-CHIKV virus, as previously described. The target gene insertion was detected by polymerase chain reaction (PCR) and polyacrylamide agarose gel electrophoresis (PAGE). The Ad5-WNV-CHIKV viral stock was purified and titrated using previously established protocols. The viral titer was expressed as IU/mL based on dilution factors.
Indirect immunofluorescence assay(IFA)
Overall, HEK 293 cells were infected with Ad5-WNV-CHIKV at 0.1 MOI. Once cytopathy reached 50–60%, the cells were fixed using a 4% tissue cell fixative (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China). Following fixation, the cells were blocked with 0.1% Triton-X and 10% goat serum (Beijing Solarbio). Then, the cells were incubated overnight at 4 °C with a rabbit monoclonal antibody against the CHIKV E2 protein (Alpha Diagnostic International, Inc., San Antonio, TX, USA) and a mouse monoclonal antibody against the WNV-E protein (40,345-MM15-100; Sino Biological, Beijing, China). After incubation, the cells were treated with FITC-labeled goat anti-rabbit immunoglobulin G (IgG) (ZF0311; ZSGB Biotech, Beijing, China) and goat anti-mouse IgG (ZF-0312; ZSGB Biotech). Finally, the stained cells were visualized using a confocal laser scanning microscope.
Western blotting(WB)
In total, HEK 293 cells were infected with Ad5-WNV-CHIKV at 0.1 MOI, and the cell lysates and supernatants were harvested after cytopathy reached 90%. The cell suspension was combined with loading buffer (Solarbio) and boiled for 10 min. Following sodium dodecyl sulfate–polyacrylamide gel electrophoresis, the proteins were transferred onto a nitrocellulose (NC) membrane. The NC membrane was blocked with 5% skim milk for 2 h, then incubated overnight at 4 °C with antibodies against CHIKV (E1,E2) or WNV(prM, E) antigens. After washing the NC membrane, it was incubated for 1 h with HRP-labeled goat anti-rabbit IgG (ZB-2301; ZSGB Biotech) or goat anti-mouse IgG (ZB-5305; ZSGB Biotech). Finally, an electrochemiluminescence luminescent solution was applied dropwise to the NC membrane, and the target protein bands were detected using imaging instruments.
Animal experiments
Female C57BL/6 mice aged 6–8 weeks were acquired from SPF (Beijing) Biotechnology Co., Ltd., China, and were housed according to the guidelines of the Institutional Laboratory Animal Care and Use Committee, approved by the Animal Ethics and Use Committee of the National Institute for Viral Disease Control and Prevention of the China Centers for Disease Control and Prevention. We administered a single 50-μL dose into the left hind leg of C57BL/6 mice by intramuscular (i.m.) injection. The mice were randomly divided into three groups. The mock group mice were immunized with Ad5-GFP at a dose of 1 × 109 vp per mouse. The low-dose group mice were immunized with Ad5-CHIKV-WNV at a dose of 2 × 108 vp per mouse. The high-dose group mice were immunized with Ad5-CHIKV-WNV at a dose of 1 × 109 vp per mouse. Serum samples were collected 2 weeks after immunization, and splenocytes were isolated to assess humoral and cellular immune responses.
Enzyme-linked immunosorbent assay
The CHIKV E1 protein (PRS-chi-004; ProSpec, Rehovot, Israel), CHIKV E2 protein (40,440-V08B100; Sino Biological), and WNV-E-DIII protein (40,345-V08Y-100; Sino Biological) were used as antigens to coat 96-well plates at a concentration of 50 ng per well. ELISA detected Antigen-specific IgG according to previously established protocols [33].
Pseudovirus neutralization test
Single-round infectious particles of WNV-SRIP were prepared as previously described [34]. Three plasmids (pCMV-D1-nluc-rep, pCAG-D1C, and WNV prM-E-expression plasmids) were provided by Professor Ryosuke Suzuki (Department of Virology II, National Institute of Infectious Diseases, Japan) for the production of WNV-SRIP. The pseudovirus neutralization test was conducted according to established methods [33] in a Biosafety Level 2 (BSL-2) laboratory. The Reed–Muench method was used to calculate the half-maximal inhibitory concentration (IC50), defined as the serum dilution that inhibited pseudovirus infection by at least 50%.
Plaque reduction neutralization test
Vero cells were seeded into 12-well plates at a density of 2 × 105 cells per well and cultured under conditions of 37 °C and 5% CO2 for 24 h. The CHIKV 181/clone25 attenuated live virus was diluted in serum-free DMEM to achieve a concentration of 20–30 PFU. Serum samples were diluted and mixed with an equal volume of virus, then incubated at 37 °C in a 5% CO2 atmosphere for 1 h. Following this, the Vero cell culture medium was removed, and the incubated virus-serum mixture was added, which was incubated for a further 1 h under identical conditions. Subsequently, the virus-serum mixture was aspirated, and a 4% Avicel covering solution was added, followed by incubation at 37 °C in a 5% CO2 chamber for 40 h. After which, the covering solution was discarded, and 4% tissue cell fixative (Solarbio) was applied to fix the cells for 30 min at room temperature. The fixative was removed, and 0.1% crystal violet solution (Solarbio) was added. Finally, the number of plaques was counted based on staining results. This process was conducted in a BSL-2 laboratory.
ELISpot
Two weeks after immunization, mice from both the vaccination and control groups were euthanized by cervical dislocation. Splenocytes were isolated from the mice, and a splenocyte suspension was prepared. CHIKV E1 (HSMTNAVTI), CHIKV E2 (IILYYYELY), and a mixture of six WNV-E protein peptides, each containing 15 amino acids (FNCLGMSNRDFLEGV, ANLAEVRSYCYLATV, VRSYCYLATVSDLST, SSAGSTVWRNRETLM, TVWRNRETLMEFEEP, IALTFLAVGGVLLFL), served as stimulating peptides, with phorbol 12-myristate 13-acetate included as a positive control. The protocol was conducted according to previously established guidelines. An ELISpot plate reader was used to quantify the number of spots per well, with the reading representing the number of interferon (IFN)-secreting T cells per million splenocytes (SFU/106 cells).
Intracellular cytokine staining
Intracellular cytokine staining (ICS) was performed as previously described [35]. In brief, spleen cell suspensions were adjusted to a concentration of 2 × 106 cells in 200 µL, and the CHIKV E1, CHIKV E2, and mixed WNV-E protein peptides were used as stimulating agents. The Fc receptor was blocked with a CD16/CD32 antibody, followed by surface staining with a mixture of CD3+-FITC, CD4+, and CD8+-APC fluorescent-conjugated antibodies. After fixation and membrane permeabilization, fluorophore-conjugated antibodies (against IFN-γ, interleukin 2 [IL-2], IL-4, and tumor necrosis factor alpha [TNF-α]) were added to the cells. Finally, the cells were resuspended in 300 µL of cell staining buffer and analyzed using flow cytometry.
Statistical analysis
GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA) was used for plotting figures and statistical analysis. The t-test or one-way analysis of variance was used for normally distributed data, and the Mann–Whitney U test was used for non-normally distributed data. The data were reported as the mean ± the standard error of the mean (SEM). P values < 0.05 were considered statistically significant.