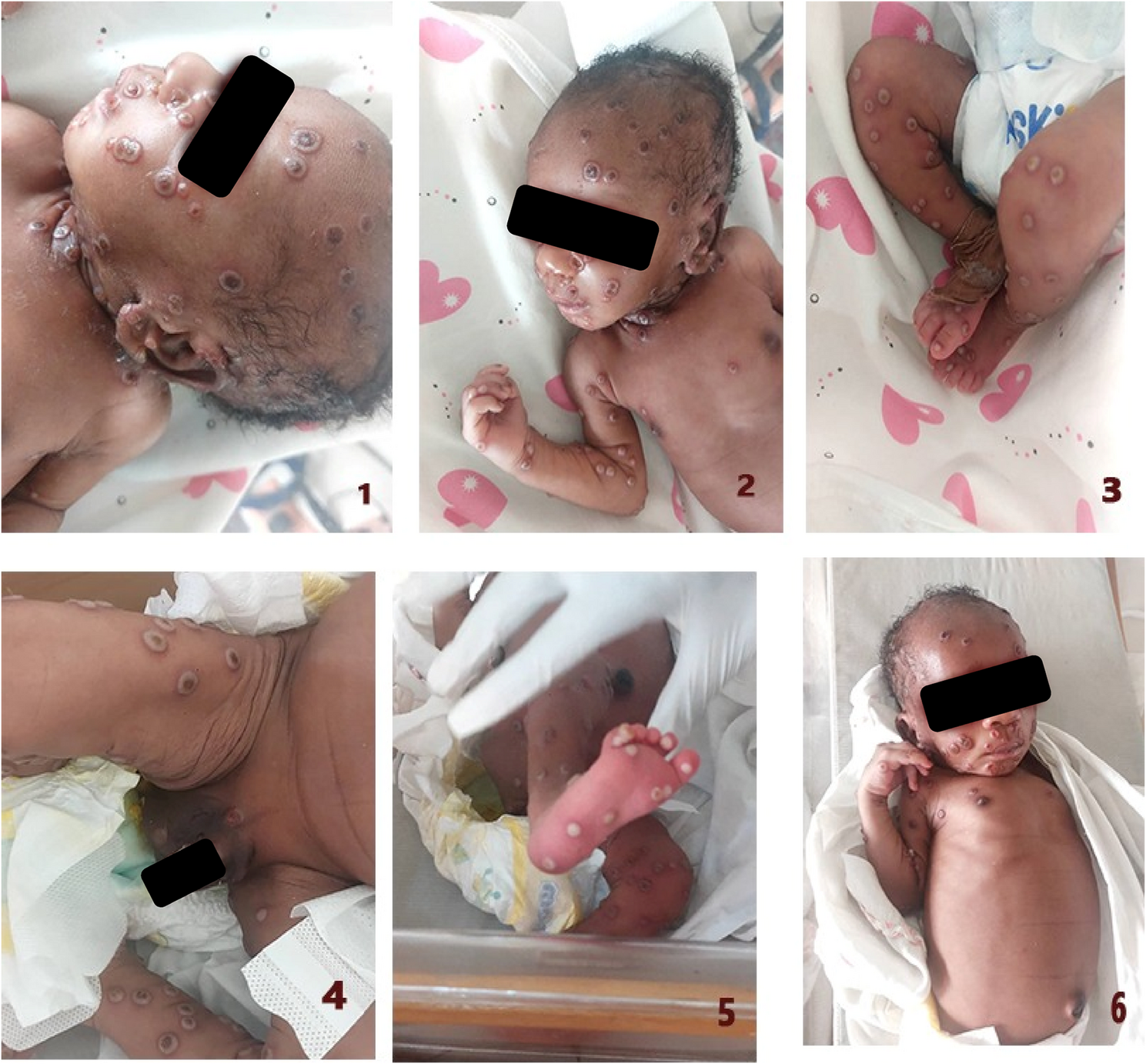
Available evidence reveals that there has been a limited number of reported cases of neonatal mpox in Nigeria [20, 21] and worldwide [22,23,24,25,26,27] despite the recognition of human mpox in 1970 and widespread demographic distribution as an endemic virus in Africa. The age group of 0–4 years only accounts for about 0.039% of documented mpox cases globally, with a paucity of information on the clinical management of mpox in this special population [28].
To the best of our knowledge, this is the first reported case of laboratory-confirmed neonatal mpox that was diagnosed in the early neonatal period (the first 7 days of life). The majority, if not all, of documented neonatal mpox in the literature were diagnosed in the late neonatal period, typically between days 8 to 10 of life, thereby creating a dilemma for clinicians and researchers on ascertaining the likely route of mpox acquisition (transplacental or postnatal transmission). This has been an area of uncertainty in the neonatal mpox disease landscape, with the majority of researchers aligning with the general categorization of a perinatal transmission of MPXV since all the documented neonatal mpox cases were diagnosed at the later stages of the newborn’s life [22,23,24,25,26,27].
Considering that our index case developed skin lesions which was confirmed as mpox on the fourth day of life and the fact that mpox has an incubation period of 5–21 days, it is therefore likely that the newborn may have been incubating the virus in utero thereby making the likelihood of a transplacental transmission of MPXV plausible. Furthermore, mpox typically begins with a prodromal phase of febrile illness, followed after 2 to 3 days by vesiculopustular skin eruptions marked on the face and limbs as well as enlarged lymph nodes. This presentation argues for a transplacental route of MPXV transmission in our patient. While postnatal exposure from the mother or the environment cannot be entirely ruled out, this is highly unlikely as the mother no longer had active skin lesions at the time of delivery and, as such, was no longer shedding the virus. Another argument in favour of postnatal transmission is that mpox infection in pregnancy tends to have adverse outcomes, including spontaneous fetal loss, stillbirth, and preterm delivery, which were not observed in this case [29]. Nevertheless, the history of maternal illness at 34th week of gestation, 3 weeks before delivery, supports an intrauterine transmission of MPXV in our patient.
The consideration of a transplacental route of MPXV transmission in this case supports earlier calls by researchers that MPXV should be included in the list of viral TORCH (an acronym for toxoplasmosis, other agents, rubella, cytomegalovirus, and herpes simplex) infection although, this requires further comprehensive fetal and maternal surveillance in pregnancies complicated by mpox [28]. A similar call was made for the inclusion of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) into the list of vertically transmitted viral infections during the coronavirus disease-2019 (COVID-19) pandemic based on documented evidence of transplacental transmission of SARS-CoV-2 to the foetus [29,30,31]. Although there is documented evidence of congenital mpox [16,17,18,19], the absence of MPXV-specific neonatal IgM, placental histopathology, in utero imaging, and maternal PCR confirmation at the time of delivery in this case weakens the hypothesis for a vertically transmitted infection. Moreover, most viral TORCH infections typically present with structural anomalies such as microcephaly, chorioretinitis, neurological sequelae, and chronic in utero infections, which were not reported in our case.
Our patient presented with the typical febrile rash syndrome characterized by a centrifugal pattern of distribution, which is pathognomonic for mpox. This is in contrast to other reported cases of neonatal mpox in the literature that reported a disseminated pattern of presentation [22,23,24,25,26,27]. Clinicians should therefore consider mpox as a differential in a newborn presenting with a vesiculo-pustular rash in addition to the traditional differential of varicella, herpes simplex, bacterial sepsis and other poxviruses such as molluscum contagiosum (in the context of a HIV exposed but uninfected newborn), especially if there is a history of similar rash in the family and an endemic region. Although varicella, the closest differential diagnosis of mpox, was negative in this case, the absence of testing for other key congenital infections is an acknowledged limitation of this paper. This underscores the diagnostic challenges in low-resource settings such as Nigeria.
The neonatal immune system is a plastic, highly adaptive system that transitions from a near-sterile environment to a plethora of new organisms after delivery [15]. Due to the immaturity of the immune system, the neonate is highly susceptible to infections that can be devastating when compared to older persons [32]. The detection of mpox in the newborn and the observation of similar symptoms in his parents also aligns with the trend that mpox transmission in Nigeria presently occurs as limited, smaller outbreaks driven by human-to-human transmission among household and non-household contacts [12, 13].
An overlooked important contributory factor to the development of poor clinical outcomes of mpox in the paediatric population is the impact of concurrent or sequential infections [33]. The development of secondary bacterial skin co-infection with MRSA, a WHO priority pathogen known for its virulence and resistance to commonly available antibiotics, may have been responsible for the persistent fever and the prolonged clinical illness in the index case evidenced by the prolonged time to skin lesion resolution of 48 days. The MRSA co-infection is likely to be of nosocomial origin since an initial wound swab microbiology done during the newborn’s admission identified no pathogen. Additionally, physiological decline and marrow suppression from sepsis could have contributed to the anaemia that was recorded in the index patient.
The management of the patient involved a multidisciplinary team of neonatologists, infectious diseases specialists, dermatologists, and other experts in clinical medicine. In line with the NCDC case management guidelines, our index case was optimized on supportive care, which included nutrition, skin care, careful attention to fluid status and fluid management, blood transfusion, and the use of targeted antibiotics to address bacterial skin MRSA co-infection. Similar to previously reported cases of neonatal mpox, our patient recovered following a prolonged clinical course and illness, indicating that timely diagnosis and institution of management potentially lead to good clinical outcomes [23,24,25,26,27].
As there are currently no completed randomized controlled trials investigating the effectiveness of mpox-specific therapeutics such as tecovirimat, brincidofovir, and cidofovir in the paediatric population, children must be prioritized in future clinical trials assessing the efficacy of novel or repurposed therapeutics for mpox. The utilization of the home-based case (HBC) model and telemedicine in the clinical management of this patient has emerged as an important adaptive approach in mpox case management, especially in resource-limited settings witnessing surge activities in their treatment centers. This is especially true given the tendency of mpox to present with non-severe disease.
In conclusion, neonatal mpox infection is rare and could lead to prolonged morbidity and mortality. Clinicians should always maintain a high index of suspicion and consider mpox in the differential diagnosis of a neonatal vesiculo-pustular rash, particularly if there is a history of similar rash in the family, as early disease recognition and early treatment are associated with improved outcomes. Further studies are needed to better understand the transmission routes of MPXV in newborns.