Elizabeth Dulau will play Mary Ann Evans, who was later known as George Eliot, while Owen Teale will play her father, Robert Evans.
Elizabeth Dulau and Owen Teale will lead the cast of Alexi Kaye Campbell’s new play Bird Grove, which opens at…

Elizabeth Dulau will play Mary Ann Evans, who was later known as George Eliot, while Owen Teale will play her father, Robert Evans.
Elizabeth Dulau and Owen Teale will lead the cast of Alexi Kaye Campbell’s new play Bird Grove, which opens at…

Matthew Koma posted a satirical Instagram response to Ashley Tisdale’s essay about leaving a ‘toxic’ mom group
Musician Matthew Koma, husband of…
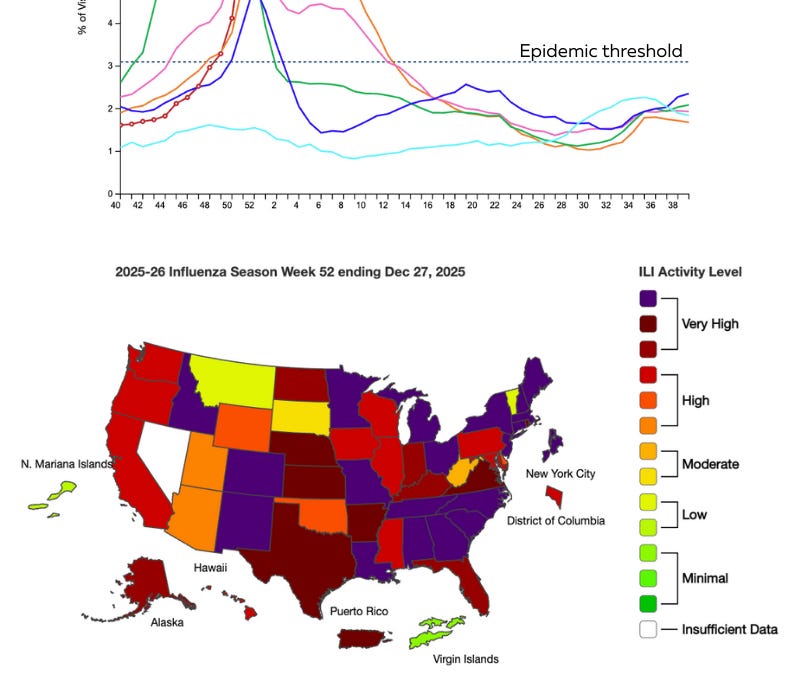
Well, it’s been a week. But happy New Year! I hope it was peaceful, magical, and restful. If you’re a parent, I’m guessing you’re just as excited as I am that school is back in session.
There’s one big thing we need to talk about: flu….

DXC is a trusted automotive partner to eight of the world’s 10 largest vehicle manufacturers, powering the future of mobility and manufacturing. DXC Luxoft software powers over 50 million vehicles and helps automakers, like Mercedes-Benz AG, Ferrari, and CARIAD, build a vehicle every three seconds. AMBER is showcased at the Consumer Electronics Show (CES) in Las Vegas from January 6-9, 2026, with live demonstrations available. Learn more about AMBER here.
About DXC Technology
DXC Technology (NYSE: DXC) is a leading enterprise technology and innovation partner delivering software, services, and solutions to global enterprises and public sector organizations — helping them harness AI to drive outcomes at a time of exponential change with speed. With deep expertise in Managed Infrastructure Services, Application Modernization, and Industry-Specific Software Solutions, DXC modernizes, secures, and operates some of the world’s most complex technology estates. Learn more on dxc.com.
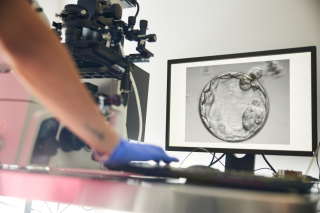
A McGill University research team has developed a painless, automated way to deliver in vitro fertilization (IVF) hormones using a light-activated microneedle patch, an innovation that could ease one of the most stressful parts of fertility…

Westminster City Council has published its City Plan Partial Review, setting out strengthened planning policies on retrofitting, affordable housing, and identifying four new, large development sites, following three years of engagement with residents, businesses, developers and local stakeholders.
At the centre of the review is Westminster’s new Retrofit First policy, placing the city at the forefront of local government action on climate change. The policy requires developers to fully explore all reasonable options for retrofitting and adapting existing buildings before seeking permission for demolition and redevelopment. It recognises that not all buildings can be retrofitted, and therefore takes a retrofit-first, not retrofit-only approach.
The urgency behind the new policy is clear: the built environment accounts for 90% of Westminster’s total CO₂ emissions, compared with around 40% for a typical local authority.
In the first half of 2025, the council’s Sustainability Team tracked the carbon performance of 19 schemes which went through planning, collectively delivering over 143,000sqm of high-quality new and refurbished office, hotel and retail floorspace. Working with applicants closely, the council has achieved a 24% reduction in construction-related carbon emissions compared to the average emissions when the policy was published in 2023. This equals 27,500 tonnes of CO2 saved, the same as the annual energy usage of nearly 3,700 homes.
In addition to its sustainability measures, the City Plan Partial Review introduces tougher requirements to deliver genuinely affordable homes. The affordable housing split in new developments will shift from 40% to 70% social rent, and from 60% to 30% intermediate homes. For the first time, sites proposing fewer than 10 homes will also be required to contribute to affordable housing delivery.
The review also identifies four strategic sites with significant potential for mixed-use development: St Mary’s Hospital, Westbourne Park Bus Garage, land adjacent to Royal Oak, and Grosvenor Sidings. These allocations provide developers and landowners with clear planning guidance to unlock new homes, modern workspaces, improved public spaces, and a new, state-of-the-art St Mary’s Hospital.
Cllr Geoff Barraclough, Cabinet Member for Planning and Economic Development, said:
“The City Plan Partial Review focuses our efforts on the most important challenges facing Westminster: tackling the climate crisis and delivering more genuinely affordable homes.
“Our Retrofit First policy sets a new benchmark for local authorities. It will help reduce carbon emissions from today’s buildings and has the potential to be the biggest single emissions-reduction initiative undertaken by any council in the country.
“We are also strengthening our commitment to affordable housing by increasing the proportion of social rent homes in new developments and ensuring smaller sites also play their part.
“Taken together, these policies create a roadmap to a fairer, healthier and more welcoming Westminster – one that works for today’s residents and for generations to come.”

Ovarian mature cystic teratomas (MCTs) are widely known as germ cell-derived neoplasms capable of multilineage differentiation.1 Although generally benign, these tumors exhibit malignant transformation in approximately 1–2% of…
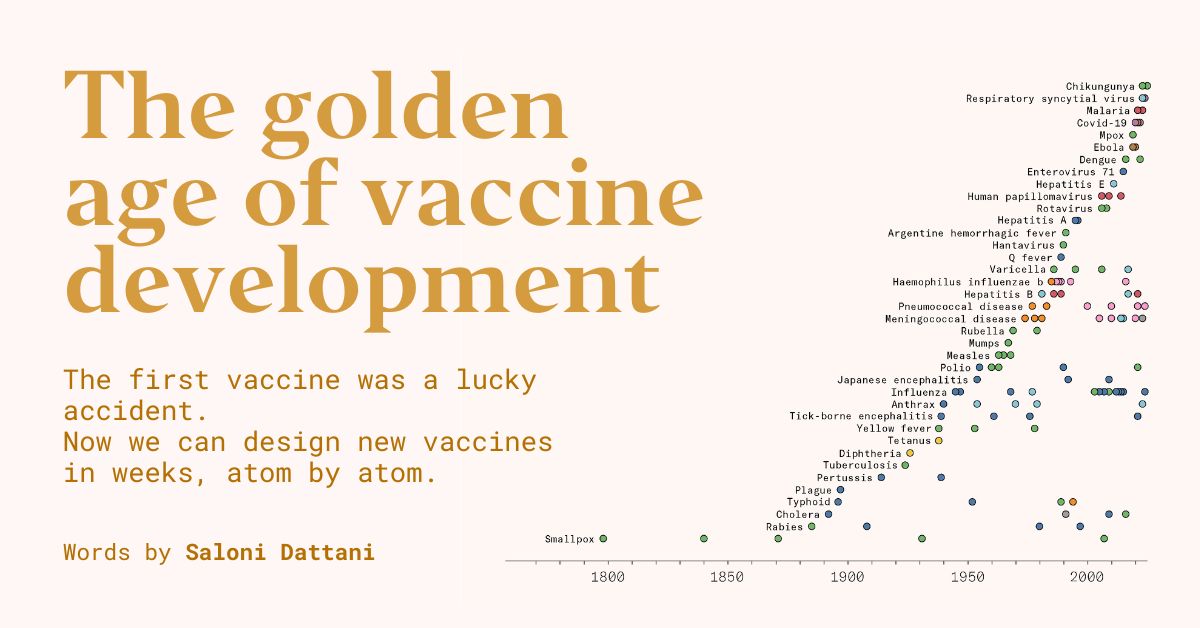
In 1796, when Edward Jenner developed the first vaccine, against the smallpox virus, no one knew what viruses were, let alone connected them to diseases.
Many believed Jenner’s vaccine worked because it depleted the body of the specific…