- Winter storms upended thousands in Gaza UN News
- Muslim bloc alarmed by deteriorating Gaza situation Dawn
- Countries demand Israel lift Gaza aid restrictions as Palestinians suffer Al Jazeera
- Gaza Humanitarian Response During Month 2 of the October…
Author: admin
-
Winter storms upended thousands in Gaza – UN News
-

Marketplace Content January 2026 | Minecraft
But enough cuteness! It’s time to reach my final form with this month’s Character Creator items, unearthing my dormant dark ice persona. To be honest, I’m somewhat unsure what that entails, but it doesn’t matter. This is the new…
Continue Reading
-

Hyundai Motor Group brings AI Robotics to life at the CES 2026
Tech Lab: Atlas and Spot demonstrate their skill
At the Tech Lab located at the center of the booth, visitors can explore Boston Dynamics’ ongoing journey of experiments and challenges to create robotics that contribute to everyday life. Designed as a research lab concept, the exhibition space features the humanoid robot Atlas and the quadruped robot Spot, both performing processes of self-learning and testing.
First, meet Boston Dynamics’ Atlas in two versions: the ‘Atlas prototype’ and the ‘Atlas product’ model. The Atlas prototype, first unveiled in 2024, was built to test core functions required for commercialization. Equipped with joints that rotate 360 degrees, it enables natural walking and efficient, stable movements, performing fully autonomous actions in work environments. In October 2025, it successfully completed pilot testing at Hyundai Motor Group’s Meta Plant America (HMGMA). Atlas repeatedly performs sequencing tasks – picking up automotive parts and aligning them in a different rack – within automotive assembly processes. Through this process, Atlas accumulates training data to continuously refine its capabilities.
Building on the Atlas prototype, we introduce a new model with enhanced efficiency and stability – the Atlas product. Leveraging Boston Dynamics’ extensive experience, Atlas product is capable of autonomous learning and adapting to any work environment, maximizing productivity in real manufacturing settings. This version features 56 DoFs(degrees of freedoms), allowing full joint rotation, and offers 360-degree vision through an integrated camera. It also boasts a maximum payload capacity of 50 kg (approximately 110 lbs), water and dustproof, and an autonomous battery-swapping function for more efficient operation. These advancements enable Atlas to learn and adapt to diverse environments and therefore is expected to significantly improv manufacturing efficiency. Hyundai Motor Group plans to mass-produce the Atlas and deploy it widely across its global manufacturing sites, including HMGMA and gradually expand its deployment through process-by-process validation.
Continue Reading
-

Elon Musk Says SpaceX Will Target Producing 10,000 Starship Rockets Annually
Commercial space flight company SpaceX‘s CEO, Elon Musk, has laid out an ambitious production target for the company’s Starship rocket.
On Sunday, Musk quoted a post on the social media platform X, which suggested that SpaceX could…
Continue Reading
-

Hyundai Motor Group Showcases AI Robotics Products and Solutions at CES 2026
At CES 2026, the Group is presenting diverse applications of AI robotics solutions which transform daily life.
Among those on display is the commercialized model of the next-generation mobility robot platform MobED, along with top-module–combined concept models developed for use in various industrial environments such as delivery and logistics. Visitors can also see various driving demonstrations showcasing its autonomous driving technology.
MobED is a compact mobility platform equipped with an innovative wheel drive system. First introduced as a concept at CES 2022, it has evolved into a commercialized model following approximately four years of development, suitable for diverse business and daily environments.
Based on Drive-and-Lift (DnL) modules, MobED features four independently driven wheels and an eccentric3 posture control mechanism. Each wheel is equipped with three motors that handle driving, steering and eccentric functions, allowing the body to tilt as needed. This enables stable balance on uneven or sloped surfaces and allows MobED to overcome curbs up to 20 cm in height.
A mounting rail on the top of MobED allows various devices to be easily attached depending on usage, while dedicated ports enable top modules to be powered and controlled using the built-in battery and controller.
The commercialized models of MobED are divided into two lineups: Basic and Pro.
The Basic model is designed for research and development purposes to implement autonomous driving robots. It serves as an experimental platform, allowing research institutions or developers who purchase the robot to apply their own custom-developed autonomous driving software as needed.
The Pro model, on the other hand, is an autonomous driving platform equipped with advanced capabilities. It features AI-based algorithms and autonomous driving technologies that integrate LiDAR and camera sensors, enabling it to recognize people and obstacles. The Pro model can safely and efficiently navigate complex and diverse environments, including indoor and outdoor spaces, for tasks such as logistics delivery, filming, and general mobility. Additionally, it includes an intuitive and user-friendly controller, allowing anyone to easily experience and operate its autonomous driving technology.
MobED measures 74 cm in width and 115 cm in length, with a maximum speed of 10 km/hour. It can operate for more than four hours of operation on a single charge and offers a maximum payload capacity ranging from 47 kg to 57 kg, depending on the lineup.
At CES 2026, the Group unveiled five conceptual models combining top modules, including MobED Pick & Place, MobED Delivery, MobED Golf, and MobED Urban Hopper, among others.
The MobED Pick & Place and MobED Delivery models are designed to support efficient delivery and logistics operations. The MobED Golf offers a hands-free, convenient golfing experience, while the MobED Urban Hopper is optimized as a scooter for urban mobility.
Additionally, the Group showcased the IONIQ5 RoboTaxi, developed in collaboration with Motional and based on the IONIQ 5.
The IONIQ 5 RoboTaxi is equipped with autonomous driving technology co-developed by the Group and Motional. It achieves Level 4 autonomy as per the standards of the Society of Automotive Engineers (SAE). Level 4 autonomy means the vehicle’s automated system can perceive, make decisions, and drive independently, even handling emergencies without requiring driver intervention.
The IONIQ 5 RoboTaxi, Motional’s first fully driverless commercial autonomous vehicle, is set to officially launch its ride-hailing service for the general public in Las Vegas, USA, starting this year.
At the exhibition, the Group is demonstrating the process of charging the IONIQ 5 RoboTaxi using its Automatic Charging Robot (ACR), as well as parking a Kia EV6 in a tight space using Hyundai WIA’s Parking Robot, capturing the attention of visitors.
The ACR features an IP65-rated waterproof and dustproof design, enabling it to operate in harsh weather conditions such as rain or snow, and in temperatures ranging from -20°C to 50°C (-4°F to 122°F). This ensures stable electric vehicle charging services even at outdoor charging stations.
Hyundai WIA’s Parking Robot is an intelligent parking system capable of moving vehicles weighing up to 3.4 tons. With its ability to control over 100 robots simultaneously, the system maximizes space utilization not only in urban parking environments but also in industrial settings.
Continue Reading
-
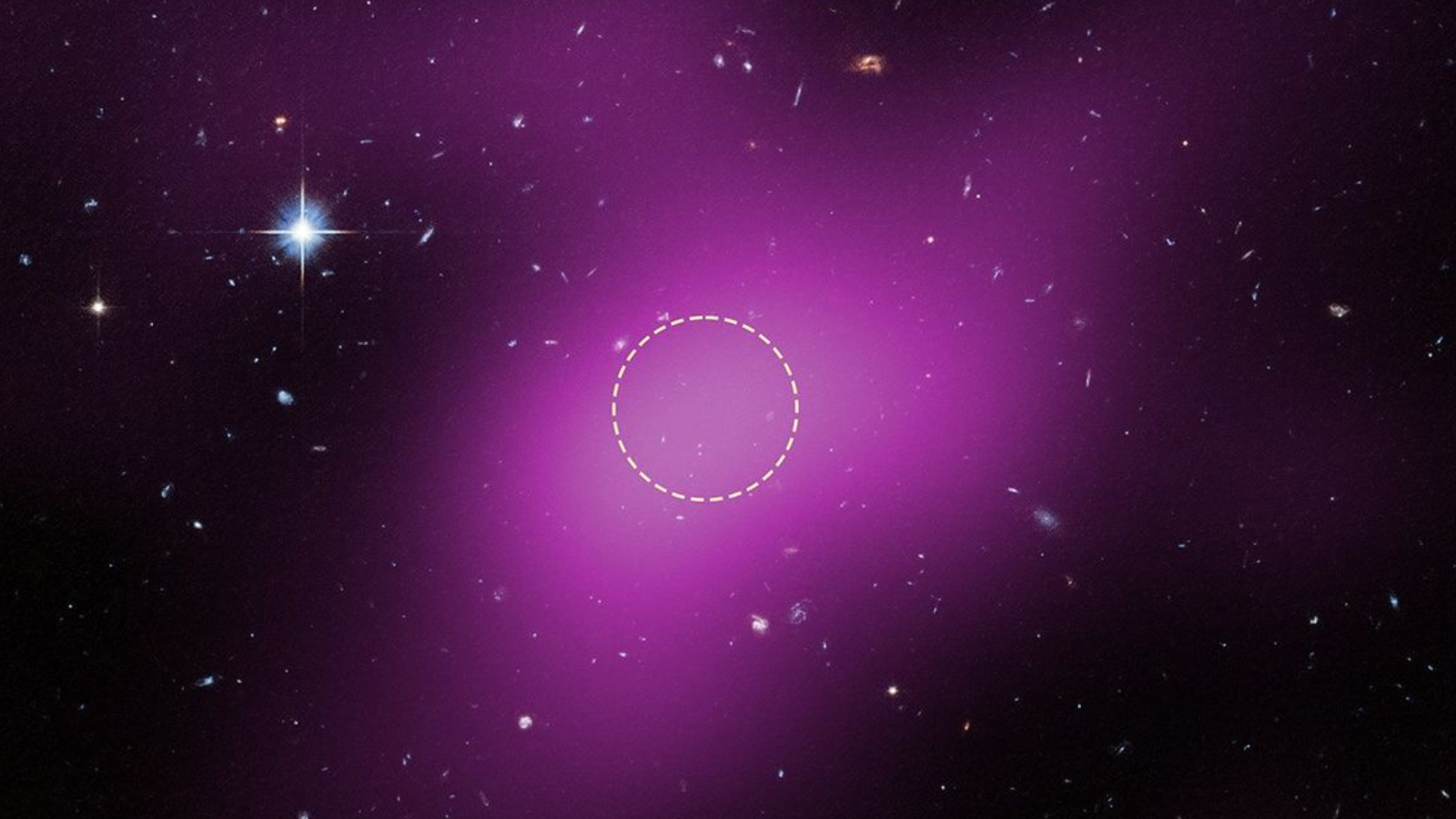
Hubble telescope discovers a new type of cosmic object and astronomers are on ‘Cloud 9’
Using the Hubble Space Telescope, astronomers have discovered a new type of cosmic object, a cloud of dark matter and gas that contains no stars. The object, located around 14 million light-years from Earth at the outskirts of the spiral galaxy…
Continue Reading
-
What you need to know > TRICARE Newsroom > TRICARE News
FALLS CHURCH, Va. –
You may have heard the terms “allowable charge” and “balance billing.” But what do these terms mean when you have TRICARE?- The TRICARE-allowable charge is the maximum amount TRICARE will pay for a procedure, service, or equipment. TRICARE-allowable charges vary based on the provider and the type, place, and date of service. The TRICARE-allowable charge determines what you’ll pay for your TRICARE cost-share.
- Balance billing is when a provider bills you for the difference between what they charge for a service (often called the “billed amount” or “billed charges”) and the TRICARE-allowable charge for that service.
Is a provider allowed to bill you for this difference? It depends on what type of provider they are.
There are two types of TRICARE-authorized providers: network providers and non-network providers. There are also two types of non-network providers: participating providers and nonparticipating providers.
“Only nonparticipating providers are allowed to practice balance billing,” said Ashli Van De Weert, health systems specialist, TRICARE Health Plan, at the Defense Health Agency. “In the U.S. and U.S. territories, these providers can’t charge you more than 15% of the TRICARE-allowable charge.”
Keep reading to learn more about different types of TRICARE-authorized providers and what they’re allowed to charge you.
A network provider is a TRICARE-authorized provider who has signed an agreement with your regional contractor to follow TRICARE policies and procedures. This means they:
- Accept a negotiated rate as payment in full
- File claims so that you don’t have to
- Won’t ask you to sign any documents to make you pay amounts above your copayment or cost-share—if this happens, contact your regional contractor.
Non-network providers are also TRICARE-authorized providers. These providers haven’t signed a formal agreement with your regional contractor. Because of this, they may see TRICARE patients on a case-by-case basis.
Participating providers are non-network providers who accept the TRICARE-allowable charge as payment in full for covered health care services. This means that after you meet your deductible, you’ll only pay a cost-share when you visit the provider. In the U.S., these providers will file claims for you.
Nonparticipating providers are non-network providers who haven’t agreed to accept the TRICARE-allowable charge as payment in full for services. They also haven’t agreed to file claims for you.
What does this mean for you?
- When you see a nonparticipating provider, you may have to pay the full amount to the provider up front and file a claim with TRICARE for reimbursement. (TRICARE won’t reimburse you for deductibles, cost-shares, and charges above the TRICARE-allowable charge.)
- In the U.S., nonparticipating providers have the legal right to charge you up to 15% more than the TRICARE-allowable charge. (This doesn’t apply if you sign a statement agreeing to pay more than the allowable charge.) This is in addition to any deductibles or cost-shares you pay.
- Overseas, there may be no limit to how much a nonparticipating provider may bill you. You’re responsible for paying any amount that’s more than the TRICARE-allowable charge.
Tip: Before you see a non-network provider, call the provider to see if they participate in TRICARE.
It’s important to make sure your provider isn’t making you pay more than they’re allowed to. This means that you should check your TRICARE explanation of benefits when you get a bill from a nonparticipating provider.
Your EOB will show you the provider’s billed amount (what they charge for the care). It will also show the TRICARE-allowable charge for the care you got, and the cost-share you paid. Your cost-share is the portion of the TRICARE-allowable charge that you’ll need to pay.
Here’s an example. A nonparticipating provider in the U.S. bills $1,000 for a service. The TRICARE-allowable charge for this service is $850. By law, the provider can ask you to pay $127.50 (15% of $850), in addition to your deductible and cost-share.
If you’re being billed for more than 15% of the allowable charge or if you’ve already paid more than this, you should call your regional contractor.
Are you deciding whether to see a network or non-network provider? Keep these things in mind.
- Out-of-pocket costs: In general, you’ll pay less out of pocket when you see a network provider than you will if you see a non-network provider. Go to Health Plan Costs to compare network and non-network costs.
- Your plan’s rules: Different TRICARE plans have different rules for seeing non-network providers. If you don’t follow these rules, you may pay more out of pocket. Go to Non-Network Providers and Book Appointments to learn more.
- Your catastrophic cap: The catastrophic cap is the most you or your family may pay out of pocket for covered TRICARE health services each calendar year. It limits the amount of out-of-pocket expenses you pay for covered services, as noted in the TRICARE Costs and Fees Sheet. However, charges above the TRICARE-allowable charge don’t count toward your catastrophic cap.
Looking for more information about TRICARE costs? Check out Cost Terms.
Would you like the latest TRICARE news sent to you by email? Visit TRICARE Subscriptions to get benefit updates, news, and more.
Continue Reading
-
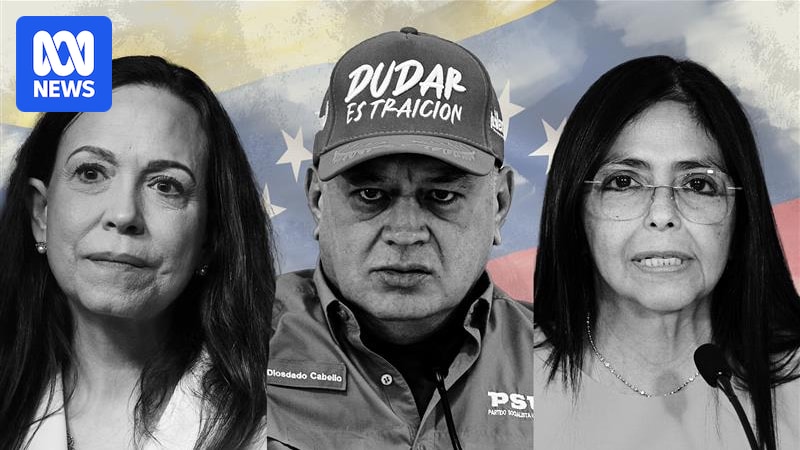
Venezuelans celebrate a future reclaimed — but insiders warn the power struggle is just getting started
For days now, Venezuelan WhatsApp, Signal and social media groups across the world have barely slept.
From Melbourne to Santiago, Miami to Madrid, phones buzzed with messages of disbelief, tears, voice notes and even cautious humour, as…
Continue Reading
-

María Corina Machado vows to return to Venezuela following ousting of Nicolás Maduro by the US
Venezuelan opposition leader María Corina Machado says she plans to return home “as soon as possible,” and has rejected the authority of the interim president who has replaced Nicolás Maduro in Caracas.
The Nobel Peace Prize winner was speaking…
Continue Reading
-

Future Coasts Aotearoa – Eos
Greetings from the Future Coasts Aotearoa (FCA) project team, all the way from New Zealand!
This picture was taken in Rangaunu Harbour, in New Zealand’s far north. We had been wading through deep mud in mangroves and salt…
Continue Reading