A groundbreaking new study reveals that changes to the gut microbiome can change the way the brain works.
Humans have the largest relative brain size of any primate, but little is known about how mammals with larger brains evolved…
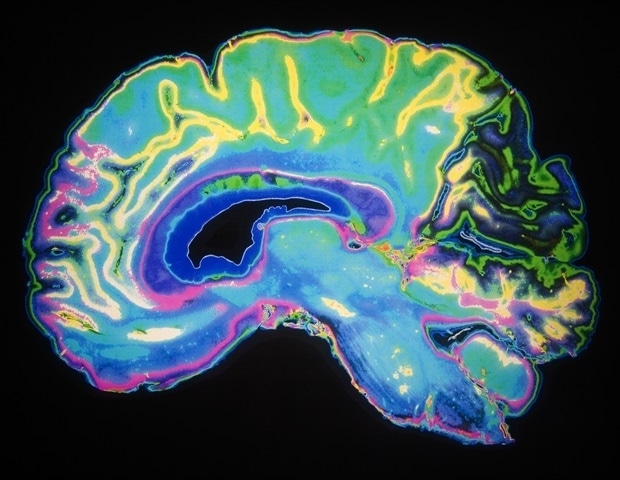
A groundbreaking new study reveals that changes to the gut microbiome can change the way the brain works.
Humans have the largest relative brain size of any primate, but little is known about how mammals with larger brains evolved…
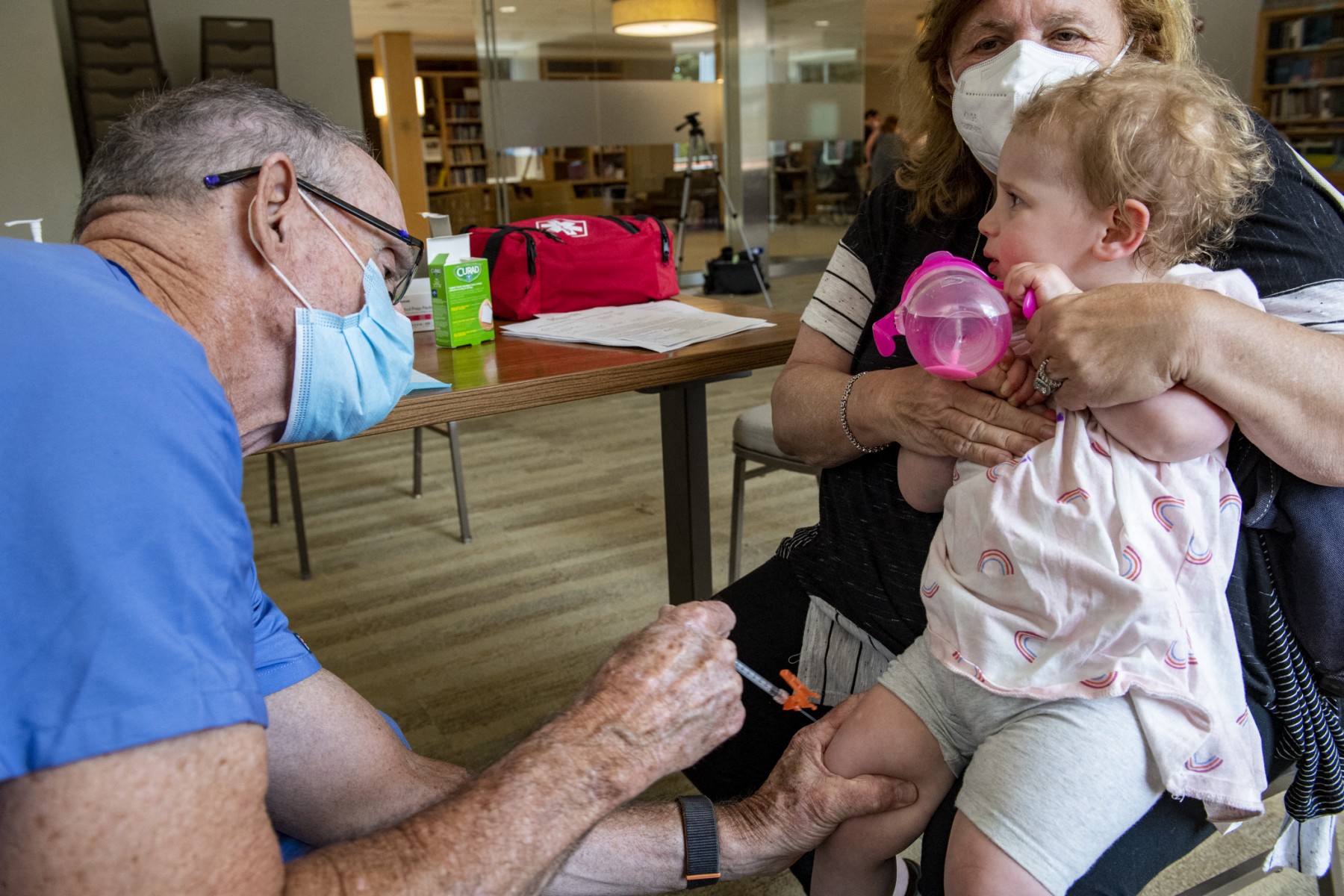
Leading medical groups in the United States have raised alarm after the Centers for Disease Control and Prevention (CDC) under President Donald Trump took the unprecedented step of cutting the number of vaccines it recommends for…

SETTING THE SCENE
Heading on the road for the first time in ACC action, Stanford men’s basketball travels to Virginia Tech on Wednesday, Jan. 7 for a 4 p.m. PT (7 p.m. ET) tip-off on ACC Network.
THE STARTING FIVE
• Stanford is out to a 12-3…

8
AI Generated Summary

The deposed Venezuelan president Nicolás Maduro pleaded not guilty to drugs, weapons and narco-terrorism charges on Monday, two days after his capture by US special forces in an operation ordered by Donald Trump that sent shockwaves around the…

SEATTLE – Washington Men’s Tennis head coach Rahim Esmail has announced his team’s 2026 spring schedule this past week. His team is looking to build upon the individual and team successes of last season and in the Fall campaign, the Huskies…