Dust. In your home, it’s a hassle. On the moon, it can be downright hazardous.
The weak gravity of the lunar surface makes it easy to kick moon dust up into floating clouds. Particles are clingy and remarkably abrasive.
On Apollo missions in…
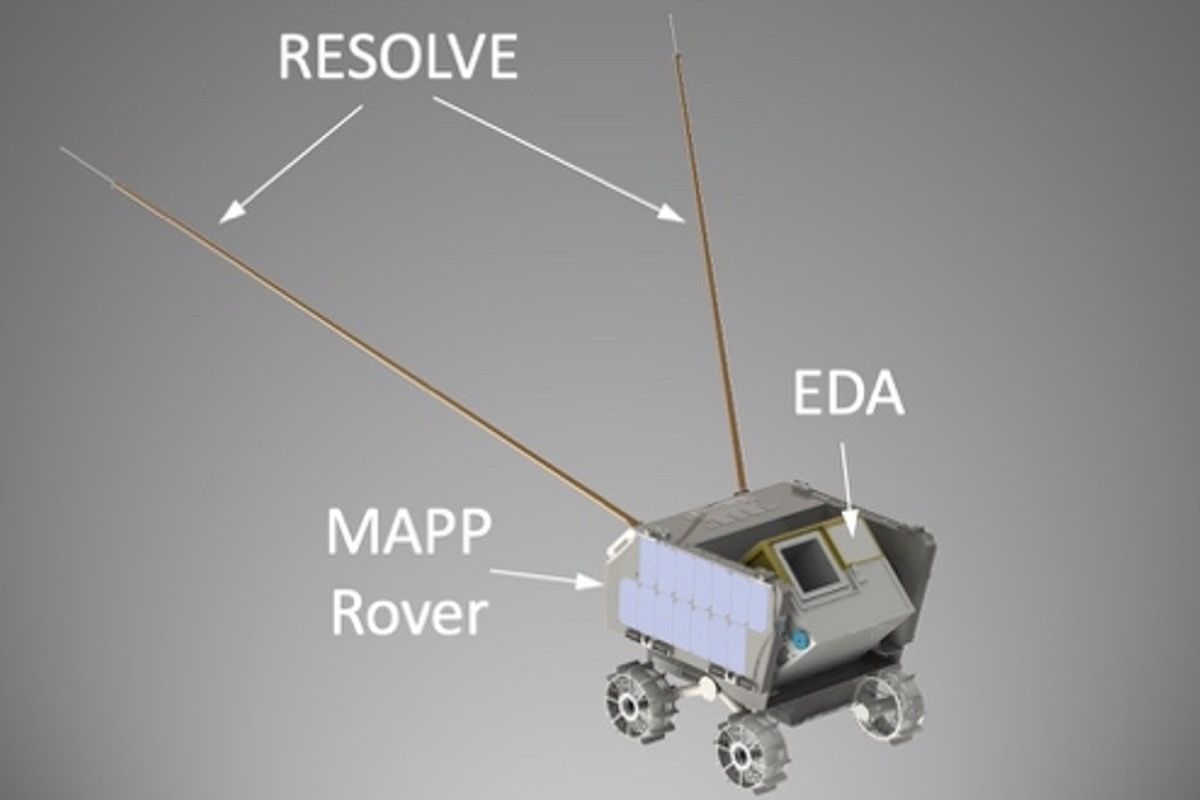
Dust. In your home, it’s a hassle. On the moon, it can be downright hazardous.
The weak gravity of the lunar surface makes it easy to kick moon dust up into floating clouds. Particles are clingy and remarkably abrasive.
On Apollo missions in…

Struggling to find a place for AI in your busy life? I understand, but I suggest taking a look at what AI features can do away from your phone or computer — and look at your home smart tech instead. Here, AI is doing some incredible work, from…
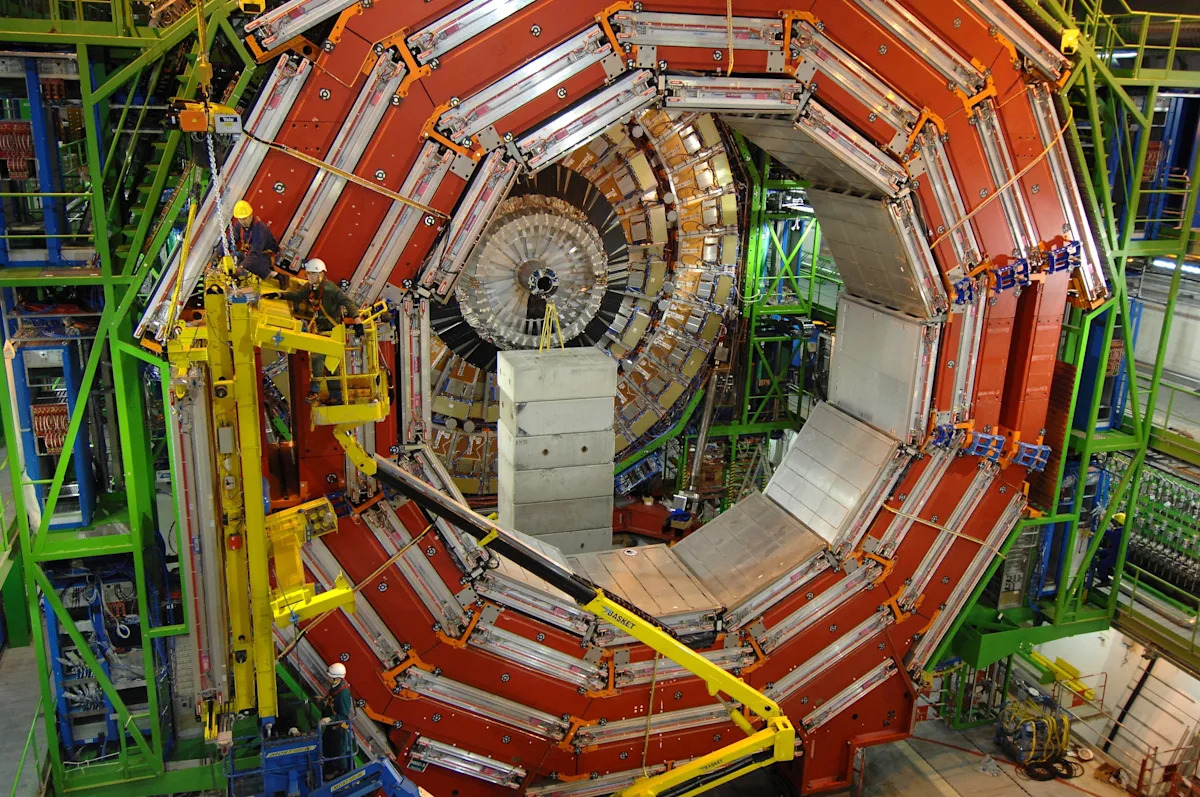
The Large Hadron Collider is going to be shut down — not permanently, but for a pretty long time — and the famous atom smasher’s eventual final retirement is also something that top scientists are now considering.
A 16-mile ring-shaped…

Islamabad court issues warrants due to non-appearance as Afridi gears up for Karachi visit on 9 Jan
Khyber-Pakhtunkhwa Chief Minister Sohail Afridi. SCREENGRAB


Greece’s Stefanos Tsitsipas played his first match in almost four months as he beat Japan’s Shintaro Mochizuki. Photo: AFP

The start of a new year is a classic time for a quiet reckoning with our health.
In fact, last January, I decided to finally look at my macros — how much protein, fiber, carbs, and other nutrients I was actually…

Australia’s stand-in captain Steve Smith has played down retirement speculation ahead of the fifth and final Test of the Ashes 2025–26 at the Sydney Cricket Ground (SCG), beginning on January 4. Touted as the greatest red-ball batter of the…

Oppo has announced the global launch of its Reno 15 series, and the lineup is now expected to make its way to Pakistan in the coming months since the Reno family of phones is quite popular here.
With smartphone prices rising sharply…