Officials in the United States have announced that the measles outbreak in the southern state of South Carolina has grown to 185 cases, up nine from earlier this week.
In Friday’s update, state officials specified that 172 of the cases involved…
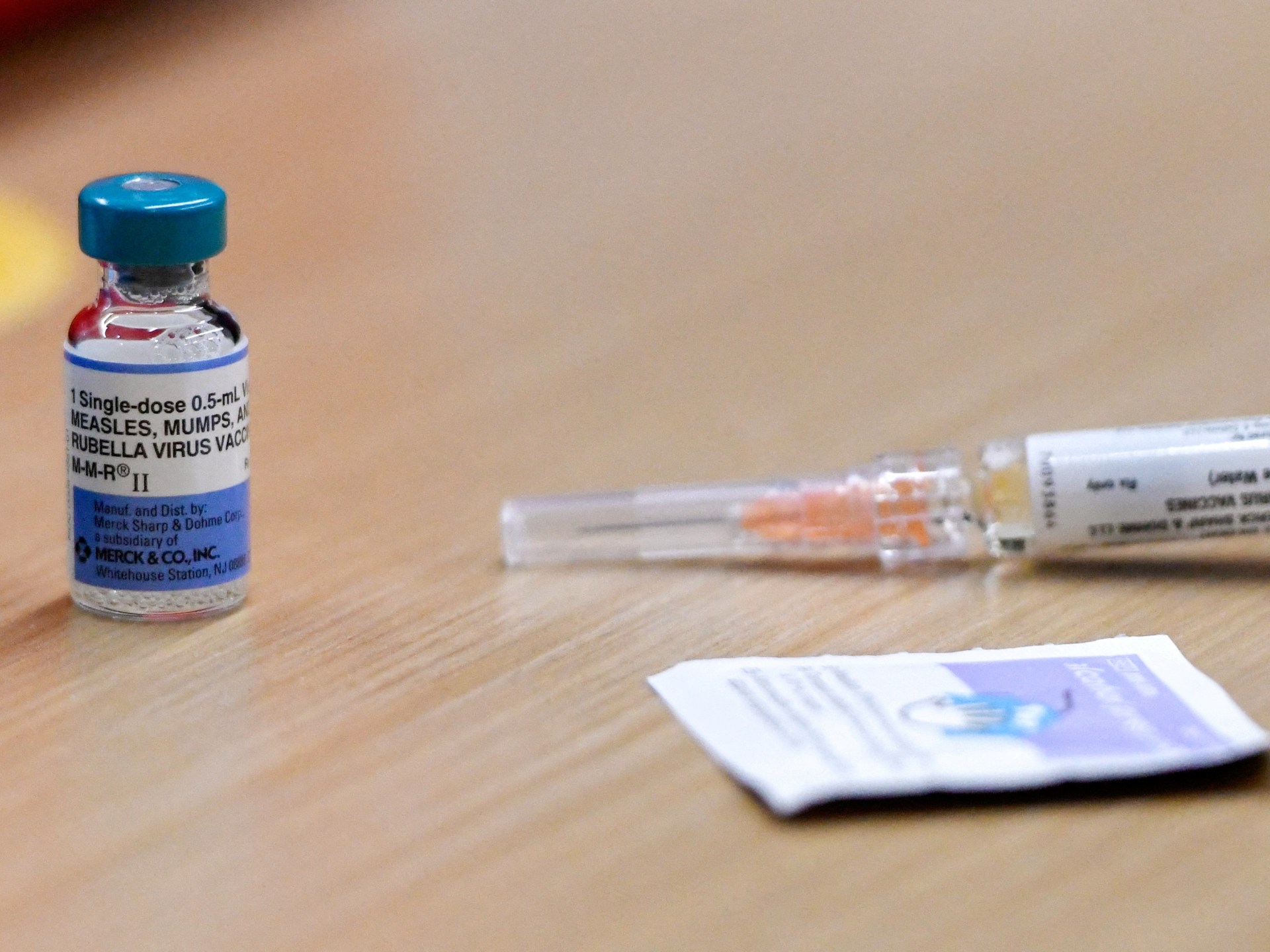
Officials in the United States have announced that the measles outbreak in the southern state of South Carolina has grown to 185 cases, up nine from earlier this week.
In Friday’s update, state officials specified that 172 of the cases involved…

Prince Harry has no plans to return to the charity founded in honor of his late…



We’ve all heard about Quitter’s Day—that infamous second Friday in January when fitness resolutions start falling apart. But here’s what’s fascinating: Apple Watch users are breaking this pattern in remarkable ways. Fresh research from Apple’s…

NASA’s Commercial Satellite Data Acquisition (CSDA) program announced eight new agreements with seven of its commercial partners, further advancing its mission to acquire data from commercial providers that support NASA’s Earth science research and applications and expand the quality, coverage, and range of Earth observation data NASA offers to the scientific community.
“These new agreements will provide users with a range of high-quality multispectral and SAR data that can be used in a variety of applications from environmental monitoring to surface deformation,” said CSDA Project Manager Dana Ostrenga. “In addition, they exemplify the CSDA program’s commitment to acquiring data that enhances and supports the agency’s application and research objectives.”
In support of NASA programs and stakeholders, the CSDA program enacted three agreements with Planet, Airbus, and Vantor (formerly Maxar) to provide near‑global multispectral and pan‑sharpened electro‑optical satellite imagery of nearly all global land and coastal surfaces. This imagery has a spatial resolution of approximately 30 centimeters, 1 meter, and up to 10 meters (depending on the product) and is suitable for applications including environmental monitoring, agriculture, and urban applications. Data products will include top of atmosphere (TOA) radiances and surface reflectance across the visible and near‑infrared spectrum.
In response to users’ need for synthetic aperture radar (SAR) data, and following rigorous technical and programmatic evaluation, CSDA executed five agreements for high‑resolution SAR imagery with Capella, ICEYE, MDA, Umbra, and Airbus. This SAR imagery includes tasked Spotlight, StripMap, Scan, Wide/Extended Spotlight, and Long‑Dwell modes, which provide all‑weather, day‑night imaging that complements the electro‑optical agreements and enhances NASA’s ability to monitor dynamic processes such as flooding, land deformation, sea‑ice motion, and infrastructure impacts. Further, under these agreements, each commercial partner will provide specific data requirements consistent with their respective instrument capabilities and performance, as well as tasking and archive access.
The data acquired under these agreements will be made available to authorized commercial satellite data users in accordance with the CSDA program’s end user license agreements (EULAs). EULAs generally pertain to NASA‑funded investigators and designated collaborators and outline established mechanisms for accessing CSDA data, such as the CSDA Satellite Data Explorer (SDX) and related portals. Users can email the CSDA program to obtain additional information about user agreements, detailed product specifications, and procedures for requesting and accessing these commercial datasets for their research and application activities.
NASA’s Earth Science Division (ESD) established the CSDA program to identify, evaluate, and acquire data from commercial providers that to support NASA’s Earth science research and applications. NASA recognizes the potential of commercial satellite constellations to advance Earth System Science and applications for societal benefit and believes commercially acquired data may also augment the Earth observations acquired by NASA, other U.S. government agencies, and NASA’s international partners.
All data from CSDA vendors are evaluated by the investigator-led CSDA project teams that assess the value of adding a vendor’s data to CSDA’s data holdings based on their quality and how they might benefit in the context of NASA Earth science research and applications.

A research team led by Max Planck Institute for Evolutionary Anthropology scientists has generated the high-quality genome assembly of a Denisovan using DNA from an ancient molar found at Denisova Cave. It belonged to a man who lived roughly…

The number of Medicare beneficiaries living with