A 5-year-old girl named Xiaoni, who underwent two high-risk cardiac surgeries over 110…
Author: admin
-
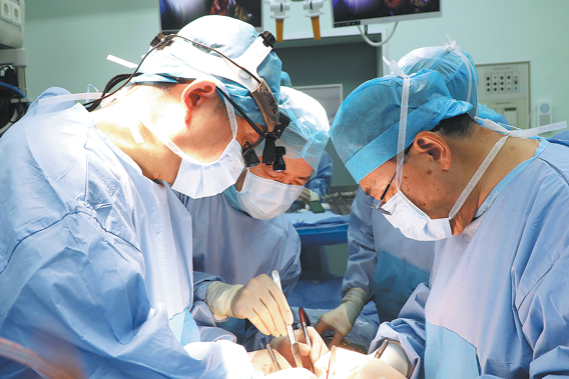
Lifesaving surgery for girl, 5, gives 2nd chance
Doctors perform a cardiac surgery on Xiaoni at TEDA International Cardiovascular Hospital in Tianjin. China Daily
-
The request could not be satisfied
ERROR: The request could not be satisfied
The request could not be satisfied.
The Amazon CloudFront distribution is configured to block access from your country.
We can’t connect to the server for this app or website at this time. There might…Continue Reading
-
Bow Wow Lounge rabies: Chicago dog daycare says dog with virus visited facility; 1st case in decades, IDPH says
CHICAGO (WLS) — For the first time in decades, a dog in Chicago tested positive for rabies.
ABC7 Chicago is now streaming 24/7. Click here to watch
The Bow Wow Lounge, a pet boarding and daycare facility on North Ravenswood said the rabid dog,…
Continue Reading
-

Orthopaedic experts share 5 foods to prevent vitamin D deficiency and maintain bone health during sun-deprived winters
Reduced sunlight exposure during winters quietly pushes a large section of the population toward vitamin D deficiency. While the issue is often underestimated, its consequences are far-reaching – ranging from bone pain and muscle weakness to
Continue Reading
-

Golden era: prices surge as rally heads towards US$5,000 in 2026, analysts forecast
Gold has hit multiple records in 2025, but analysts believe the rally is far from over, with some forecasting the yellow metal could climb to US$5,000 per ounce amid geopolitical tensions and a buying spree by central banks.
Spot gold broke through the US$4,500-per-ounce mark for the first time, reaching a record US$4,510 on Christmas Eve on Wednesday, which was 72 per cent higher than the end of last year, when it stood at US$2,624.
This was the biggest annual jump for the precious metal, exceeding the 70 per cent rise in 1979, according to Brian Fung, CEO of the Hong Kong Gold Exchange. The increase followed a 26 per cent surge in 2024.Local prices rose in tandem, with gold in Hong Kong hitting a record HK$41,855 (US$5,382) per tael (37.51 grams) on Monday, according to the exchange.
Fung expected the rally to continue in 2026, with prices potentially hitting US$5,000 per ounce.
“The gold rally in 2025 was driven by expectations of interest-rate cuts, geopolitical tensions, and tariffs introduced by US President Donald Trump,” Fung told the Post. “Individual investors and central banks wanted to diversify away from US dollar assets, and gold became a safe-haven alternative.”Continue Reading
-

2 police officers killed in Moscow explosion
Russian investigative authorities say two police officers and a “suspicious individual” were killed in an explosion in Moscow.
Russia’s Investigative…
Continue Reading
-
Exploiting terrorism for political ends unacceptable: army – Business Recorder
- Exploiting terrorism for political ends unacceptable: army Business Recorder
- Military top brass says no malicious interest, political or otherwise, will be tolerated Dawn
- CDF Munir warns of proxy, hybrid threats The Express Tribune
- Pakistan army…
Continue Reading
-
American Sikhs laud implementing of Sikh Marriage Act: Maryam vows to ensure safety, comfort & convenience of Sikh pilgrims visiting Punjab – Business Recorder
- American Sikhs laud implementing of Sikh Marriage Act: Maryam vows to ensure safety, comfort & convenience of Sikh pilgrims visiting Punjab Business Recorder
- US Sikh delegation applauds Punjab for landmark Sikh Marriage Act The Express Tribune
Continue Reading

 KARACHI : Despite mob violence and threat of a direct gun attack, K-Electric (KE) conducted a massive crackdown on electricity theft in Karachi’s SITE area where a loadshed-exempted industrial feeder was being used to steal over 10…
KARACHI : Despite mob violence and threat of a direct gun attack, K-Electric (KE) conducted a massive crackdown on electricity theft in Karachi’s SITE area where a loadshed-exempted industrial feeder was being used to steal over 10…