A new battery technology has been developed that delivers significantly higher energy storage—enough to alleviate EV range concerns—while lowering the risk of thermal runaway and explosion. A research team at POSTECH has developed a…
Author: admin
-

Nano-enhanced Diets: Advancing Metabolic Dysfunction-Related Steatotic
Introduction
The liver plays a crucial role in protein synthesis, glucose and lipid metabolism, and detoxification.1 Metabolic-associated fatty liver disease (MAFLD), previously known as non-alcoholic fatty liver disease (NAFLD), was recently…
Continue Reading
-

AI-powered wearable boosts preventative care for elderly
University of Arizona researchers in the Gutruf Lab have developed a comfortable, easy-to-use wearable device that incorporates artificial intelligence to detect subtle warning signs of frailty, signifying a leap forward in elderly care.
“The…
Continue Reading
-
How to avoid scams and beat the fraudsters this festive shopping season – Microsoft Source
- How to avoid scams and beat the fraudsters this festive shopping season Microsoft Source
- ‘Game of cat and mouse’ – What happens when you call a scammer? RTE.ie
- Gifts that don’t exist and fake invoices to busy staff: Scams doing the rounds this Christmas The Journal
- Lidl and Tesco among leading retailers being impersonated by fraudsters in social media ad scams The Irish Times
- Scam social media ads impersonating retailers increase over Christmas period, bank warns laois-nationalist.ie
Continue Reading
-
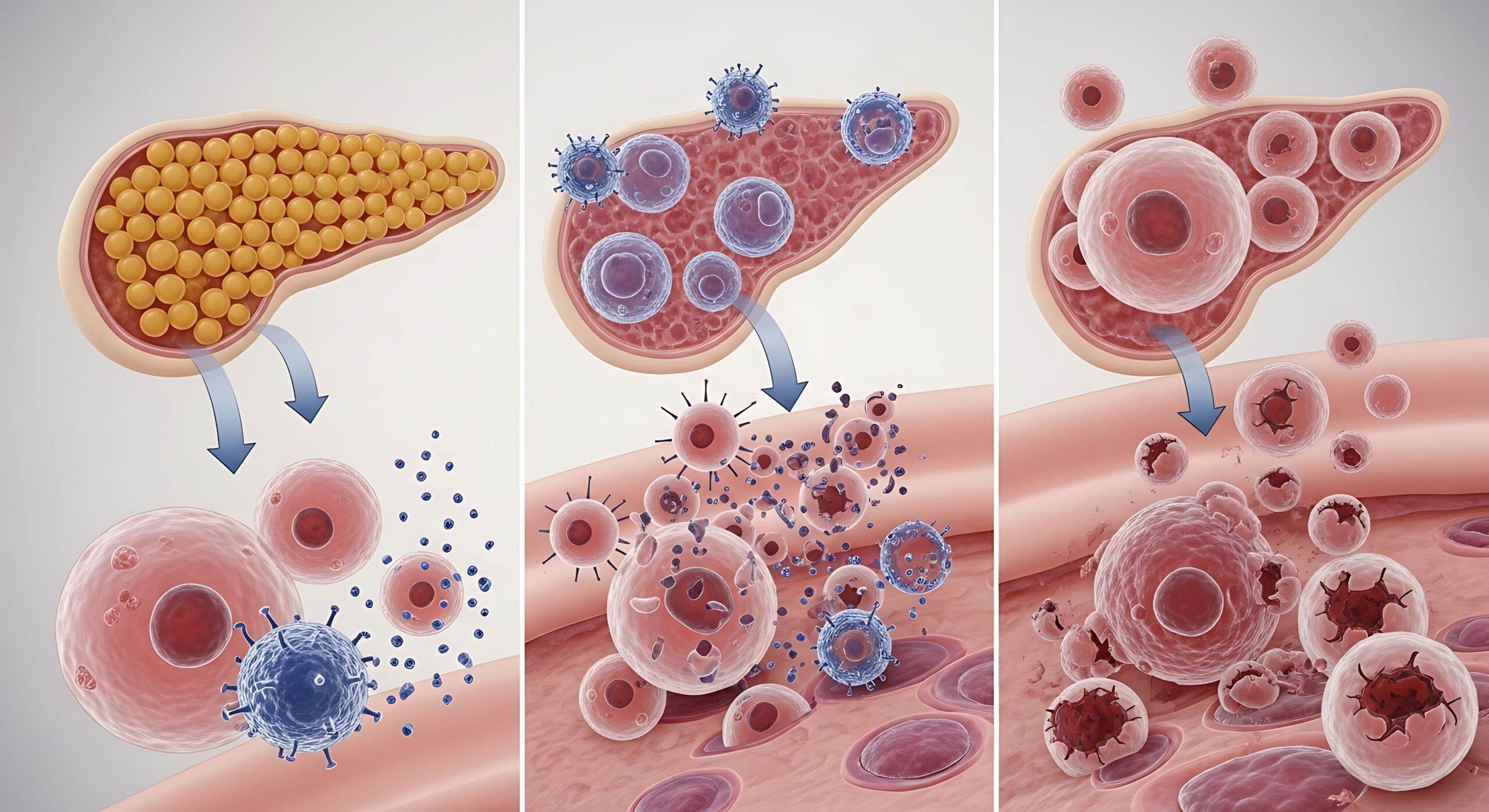
Immune system targets single beta cells before Type 1 diabetes symptoms show
Researchers from the University of Florida’s Diabetes Institute have found an important clue for why the body’s immune system destroys insulin-producing cells in the pancreas, which leads to Type 1 diabetes. They found that single beta cells…
Continue Reading
-

The Solar System Loses an Ocean World
Saturn’s largest moon, Titan, may not have a subsurface ocean after all.
That’s according to a re-examination of data captured by NASA’s Cassini spacecraft, which flew by Titan dozens of times starting in 2004. By 2008, all the…
Continue Reading
-

DVIDS – News – Line? What Line? Army & Air Force Exchange Service Premieres Online Ticketing, Seat Selection for Reel Time Theaters
Line? What Line? Army & Air Force Exchange Service Premieres Online Ticketing, Seat Selection for…Continue Reading
-

Volleyball Adds Transfer Štiglic – Northwestern Athletics
EVANSTON, Ill. – Northwestern volleyball has added undergraduate transfer Mara Štiglic to the roster ahead of winter quarter, Head Coach Tim Nollan announced on Monday. Štiglic, a sophomore, will join the Wildcats after two seasons at Utah…Continue Reading
-
Pakistan committed to empowering youth, creating transformative opportunities-Xinhua
ISLAMABAD, Dec. 22 (Xinhua) — Chairman of the Pakistani Prime Minister’s Youth Program Rana Mashhood Ahmad Khan said that the country is committed to empowering youth and creating transformative opportunities through inclusive policies,…
Continue Reading
-
Pakistan committed to empowering youth, creating transformative opportunities-Xinhua
ISLAMABAD, Dec. 22 (Xinhua) — Chairman of the Pakistani Prime Minister’s Youth Program Rana Mashhood Ahmad Khan said that the country is committed to empowering youth and creating transformative opportunities through inclusive policies,…
Continue Reading
