When costume designer Julian Day took on F1 The Movie, he faced a unique challenge – creating and kitting out an entire Formula 1 team in just a few months.
Most teams spend years developing their identity, uniforms, and brand – from their…

When costume designer Julian Day took on F1 The Movie, he faced a unique challenge – creating and kitting out an entire Formula 1 team in just a few months.
Most teams spend years developing their identity, uniforms, and brand – from their…

After a one-year layoff, Tampa’s Gasparilla Music Festival is returning in 2026 — but at a different downtown venue.
In a social media post Wednesday, organizers announced the 14th edition of the festival…

Musculoskeletal disorders are one of the leading causes of disability worldwide.1 According to the Global Burden of Disease (GBD) Study 2017, they are the second-highest contributors to global disability-adjusted life years (DALYs),…
December 18, 2025
News Release 25-127
Inv. No(s).
337-TA-1468
Contact: Claire Huber
, 202-205-1819
USITC Institutes Section 337 Investigation of Certain Smart Wearable Devices, Systems, and Components Thereof
The U.S. International Trade Commission (Commission or USITC) voted to institute an investigation of certain smart wearable devices, systems, and components thereof. The products at issue in the investigation are described in the Commission’s notice of investigation.
The investigation is based on a complaint filed on behalf of Ouraring Inc. of San Francisco, California on November 18, 2025. An amended complaint was filed on December 9, 2025. The amended complaint alleges violations of section 337 of the Tariff Act of 1930 in the importation into the United States and sale of certain smart wearable devices, systems, and components thereof that infringe certain claims of the patents asserted by the complainants. The complainant requests that the USITC issue a limited exclusion order and cease and desist orders.
The USITC has identified the following respondents in this investigation:
By instituting this investigation (337-TA-1468), the USITC has not yet made any decision on the merits of the case. The USITC’s Chief Administrative Law Judge will assign the case to one of the USITC’s administrative law judges (ALJ), who will schedule and hold an evidentiary hearing. The ALJ will make an initial determination as to whether there is a violation of section 337; that initial determination is subject to review by the Commission.
The USITC will make a final determination in the investigation at the earliest practicable time. Within 45 days after institution of the investigation, the USITC will set a target date for completing the investigation. USITC remedial orders in section 337 cases are effective when issued and become final 60 days after issuance unless disapproved for policy reasons by the U.S. Trade Representative within that 60-day period.
# # #
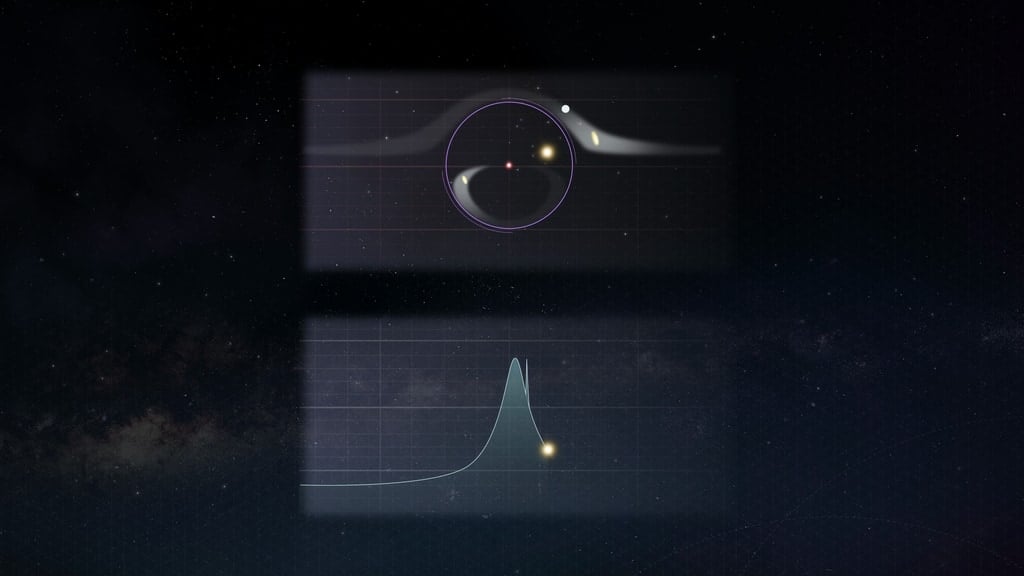
With new technologies comes new discoveries. Or so Spider Man’s Uncle Ben might have said if he was an astronomer. Or a scientist more generally – but in astronomy that saying is more true than many other disciplines, as many…


Posted on Thursday 18th December 2025
Students at a Staffordshire college have explored their vision for the shaping of a town centre in a workshop event this week.
They were joined by graduate urban designers from a company…

Listening to your tunes, but your neighbor is feeling chatty? Ordering a latte but your hands are full so you can’t pause your podcast? Conversation detection, a feature on some headphones and earphones, can be a game-changer. Instead of removing…