- Machado Won’t Pick Up Peace Prize in Person, Nobel Director Says The New York Times
- Nobel laureate’s whereabouts unknown ahead of ceremony Dawn
- Machado will be in Oslo for Nobel Peace Prize, Nobel institute says Reuters
- Nobel officials unsure…
Author: admin
-
Machado Won’t Pick Up Peace Prize in Person, Nobel Director Says – The New York Times
-

Pakistan’s progress lies in protection of human rights: Abbasi
Federal Minister for Railways Muhammad Hanif Abbasi Wednesday emphasized that the path to Pakistan’s progress lies in the complete protection and promotion of human rights.
In his message…Continue Reading
-
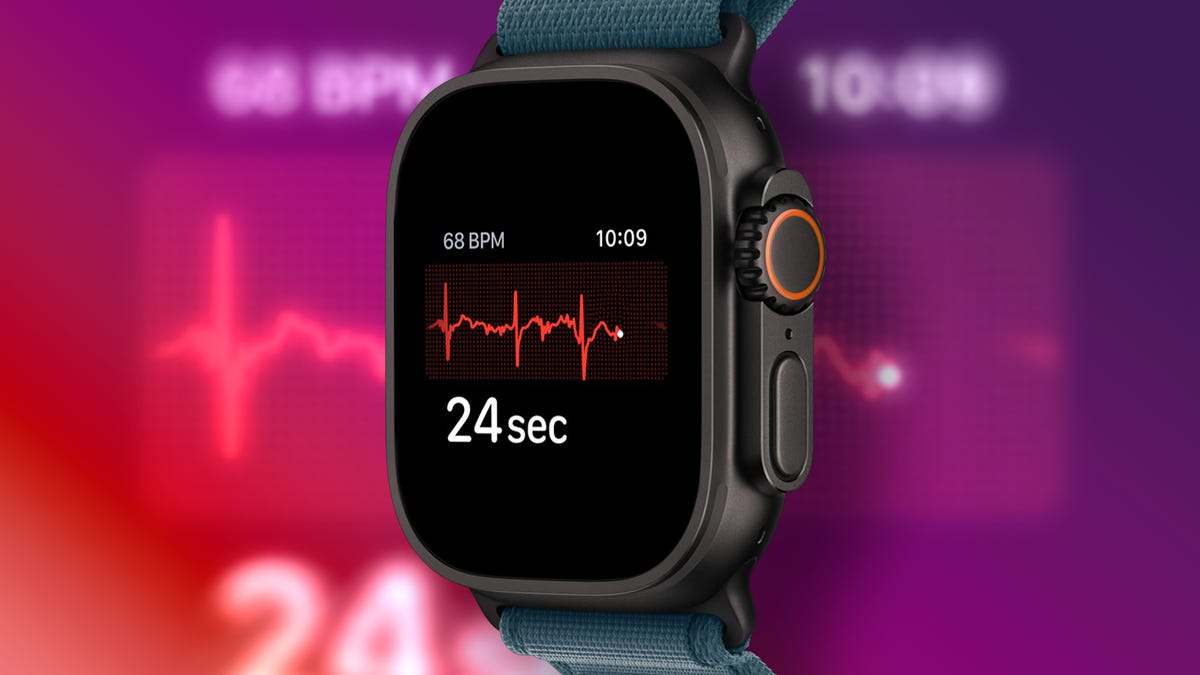
A Doctor at Apple Reveals 9 Hidden Apple Watch Health Features
An Apple Watch can be used for countless tasks — making phone calls, responding to text messages and reading emails, to name just a few — but did you know that this smartwatch is also a powerhouse when it comes to monitoring and supporting your…
Continue Reading
-
Ascletis Announces China National Medical Products Administration Acceptance of New Drug Application for Denifanstat (ASC40), a First-in-Class FASN Inhibitor for Acne Treatment USA – English APAC – English APAC – Traditional Chinese
-Denifanstat (ASC40) met all primary, key secondary and secondary efficacy endpoints (ITT analysis) and significantly improved moderate-to-severe acne vulgaris compared with placebo in a randomized, double-blind, placebo-controlled, multicenter Phase III clinical trial.
HONG KONG, Dec. 10, 2025 /PRNewswire/ — Ascletis Pharma Inc. (HKEX: 1672, “Ascletis”) announces today that its New Drug Application (NDA) for denifanstat (ASC40), a first-in-class, once-daily oral small molecule fatty acid synthase (FASN) inhibitor for the treatment of moderate-to-severe acne vulgaris, has been accepted by the China National Medical Products Administration (NMPA).
“Acceptance of this NDA is an important milestone in our efforts to provide a potentially groundbreaking therapeutic approach for the treatment of moderate-to-severe acne,” said Jinzi Jason Wu, Ph.D., Founder, Chairman and CEO of Ascletis, “We are excited denifanstat (ASC40) is only one step away from the commercialization.”
Ascletis has completed Phase II (NCT05104125) and Phase III (NCT06192264) studies of denifanstat (ASC40) for the treatment of moderate-to-severe acne vulgaris.
In the Phase III study, denifanstat (ASC40) met all primary, key secondary and secondary efficacy endpoints (ITT analysis) and significantly improved moderate-to-severe acne vulgaris compared with placebo. Denifanstat (ASC40) demonstrated a favorable safety and tolerability profile. All denifanstat (ASC40)-related treatment-emergent adverse events (TEAEs) were mild (Grade 1) or moderate (Grade 2). There were no denifanstat (ASC40)-related Grade 3 or 4 TEAEs and no denifanstat (ASC40)-related serious adverse events (SAEs). There were no denifanstat (ASC40)-related permanent treatment discontinuations or withdrawals observed.
The Phase III study results were presented as an oral presentation at the European Academy of Dermatology and Venereology (EADV) Congress 2025 in Paris, France on September 17, 2025 (link).
The Company recently completed the pre-NDA consultation with the China NMPA for denifanstat (ASC40) for the treatment of moderate-to-severe acne vulgaris and received positive feedback from NMPA.
Ascletis licensed denifanstat (ASC40) from Sagimet Biosciences Inc. (Nasdaq: SGMT) for exclusive rights in Greater China.
About Ascletis Pharma Inc.
Ascletis Pharma Inc. is a fully integrated biotechnology company focused on the development and commercialization of potential best-in-class and first-in-class therapeutics to treat metabolic diseases. Utilizing its proprietary Artificial Intelligence-Assisted Structure-Based Drug Discovery (AISBDD) and Ultra-Long-Acting Platform (ULAP) technologies as well as Peptide Oral Transport ENhancement Technology (POTENT), Ascletis has developed multiple drug candidates in-house, including both small molecules and peptides, such as its lead program, ASC30, a small molecule GLP-1R agonist designed to be administered once daily orally and once monthly to once quarterly subcutaneously as a treatment therapy and a maintenance therapy for chronic weight management; ASC36, a once-monthly subcutaneously administered amylin receptor peptide agonist, ASC35, a once-monthly subcutaneously administered GLP-1R/GIPR dual peptide agonist and ASC37, an oral GLP-1R/GIPR/GCGR triple peptide agonist for chronic weight management. Ascletis is listed on the Hong Kong Stock Exchange (1672.HK).
For more information, please visit www.ascletis.com.
Contact:
Peter Vozzo
ICR Healthcare
443-231-0505 (U.S.)
[email protected]Ascletis Pharma Inc. PR and IR teams
+86-181-0650-9129 (China)
[email protected]
[email protected]SOURCE Ascletis Pharma Inc.

Continue Reading
-

Nnena Kalu ‘makes history’ by winning Turner Prize 2025
A British Nigerian artist who produces large-scale draped sculptures and vortex-like circular drawings has become the first person with a learning disability to claim the Turner Prize, one of contemporary…
Continue Reading
-
We welcome FDA moves to cut primate testing as roadmap begins to take shape – Cruelty Free International
- We welcome FDA moves to cut primate testing as roadmap begins to take shape Cruelty Free International
- Simulations Plus Positioned to Capitalize on FDA’s Streamlined Nonclinical Safety Guidance with Adva pharmiweb.com
- CDC Seeks to End Program Using Monkeys in Research Animal Legal Defense Fund
- FDA takes steps to limit non-human primate testing Pharmaphorum
- FDA Endorses Non-Animal Methods for Drug Development, Supporting PETA Scientists’ Diphtheria Therapy Strategy PETA
Continue Reading
-
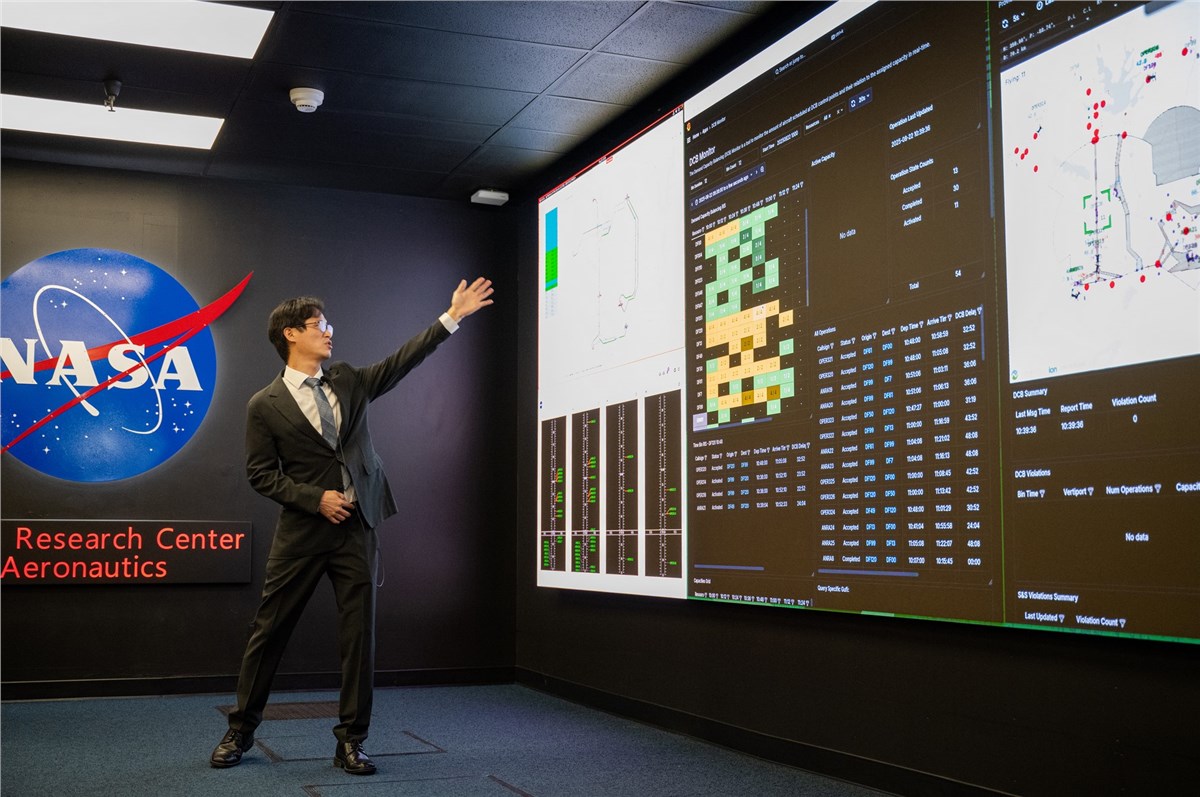
NASA Demonstrates Safer Skies for Future Urban Air Travel
NASA is helping shape the future of urban air travel with a new simulation that will manage how electric air taxis and drones can successfully operate within busy areas.
The demonstration, held at NASA’s Ames Research Center…
Continue Reading
-
Cell Signaling Regulation Uncovered in cAMP Pathways
When a cell receives a message from outside, it generates a molecule called cyclic AMP (cAMP) to relay this message. To ensure the signal reaches the correct effector without triggering pathways accidentally, cAMP levels must be maintained around…
Continue Reading
-

Rolls-Royce celebrates the official opening of BAESL, its MRO joint venture in Beijing, China
Air China, as China’s sole national flag carrier, is a long-term strategic customer for Rolls-Royce. By establishing BAESL jointly, the two companies have deepened their cooperation. In the meantime, it further enhances Air China’s strategic layout in the aircraft maintenance industry chain, improving its overall fleet operational support capabilities.
As one of four authorised joint-venture overhaul facilities within the Rolls-Royce global services network, BAESL will be an integral part of its worldwide Trent MRO ecosystem, which also consists of two Rolls-Royce maintenance facilities, seven joint-venture or independent Authorised Maintenance Centres, and customer-owned shops.
Starting from 2026, BAESL will begin introducing overhaul capability for Trent 700, Trent XWB-84 and Trent 1000 engines, with capacity expected to ramp up to 250 overhauls per year by 2034.
Continue Reading
-
How to Build an AI App from Scratch With Zero Coding Skills
Thanks to the Generative AI boom, building an app or website is now just a text prompt away. You don’t have to learn coding or various languages to build a personal app for you. Vibe coding has really taken off and now you can build an…
Continue Reading