Researchers have discovered how to inhibit the P2X4 receptor, a key protein linked to chronic pain, inflammation and certain cancers.

Scientists at the University of Bonn and University Hospital Bonn (UKB) have discovered a…

Researchers have discovered how to inhibit the P2X4 receptor, a key protein linked to chronic pain, inflammation and certain cancers.

Scientists at the University of Bonn and University Hospital Bonn (UKB) have discovered a…
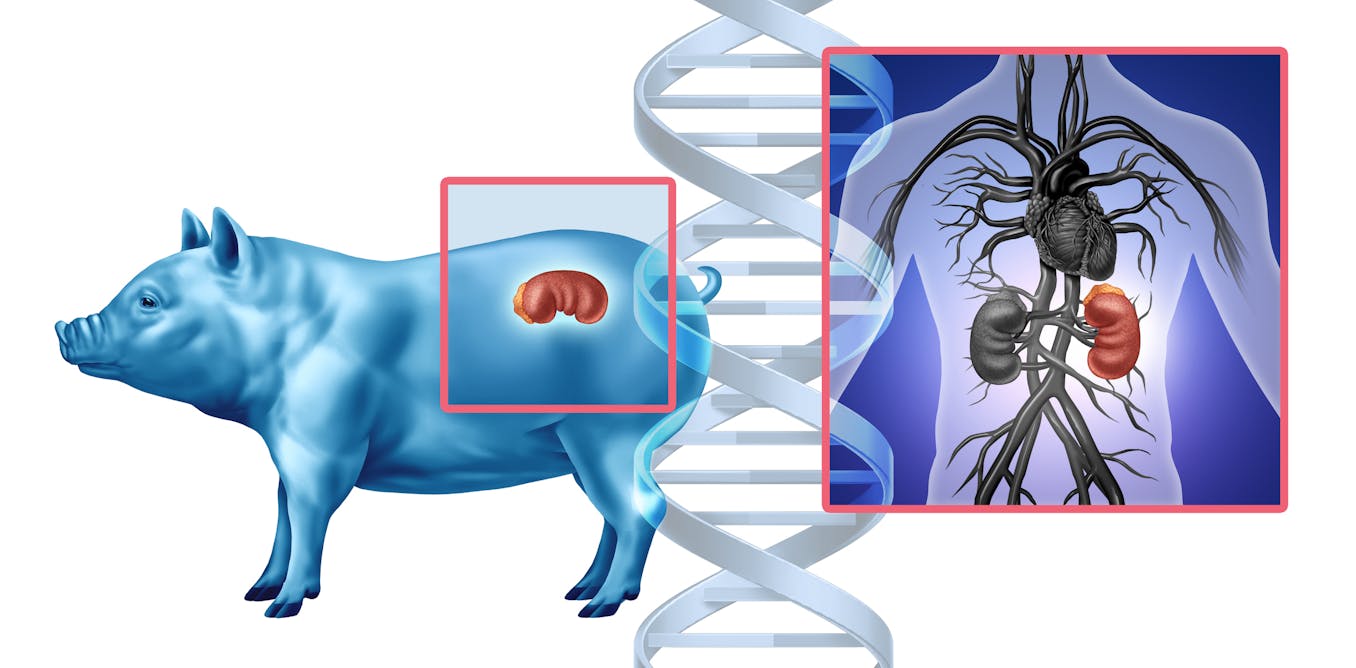
In a Maryland operating room one day in November 2025, doctors made medical history by transplanting a genetically modified pig kidney into a living patient. The kidney had been engineered to mimic human tissue and was grown in a pig, as an…

For millennia, the act of gazing at the night sky has connected us not only to the stars but also to one another. This simple, shared experience ignites our curiosity, inspiring philosophical and scientific quests to peer deep into the…

The man with the Napoli tattoo was met with hostile whistles on his return to the Stadio Maradona. Luciano Spalletti had the club’s emblem inked on to his arm, together with a Scudetto badge, after leading the Partenopei to their third Serie A…
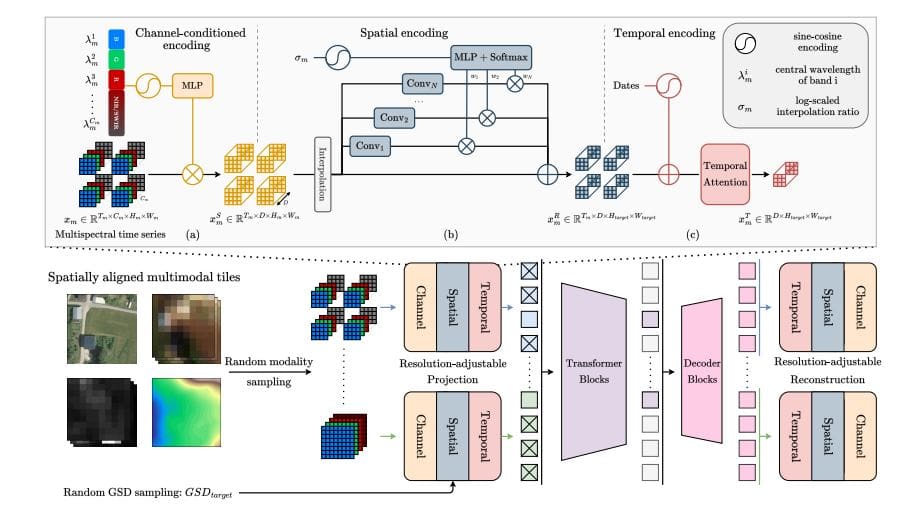
Earth observation generates data with vastly different levels of detail, ranging from sharp images to broad, low-resolution scans, creating challenges for comprehensive analysis. Nicolas Houdré from Université Paris Cité, Diego Marcos and…

The Indian cricket team, bolstered by the return of Shubman Gill and Hardik Pandya, will square off against South Africa in a five-match T20I series at home, which starts on Tuesday.
The first and second matches of the series are set to be held…

The viral clip of Liverpool’s media manager reacting to Mohammed Salah’s explosive mixed zone interaction on Saturday struck a chord over in Brisbane.
Just under 10,210 miles separate Elland Road and the Gabba, where England head coach Brendon…

Read more:
Some 31% of kids still live in poverty, the same proportion who did so in 1989. Trussell Trust distributed 2.9 million emergency food parcels in 2024/25, a dramatic increase from the 60,000 parcels distributed in 2010/11….