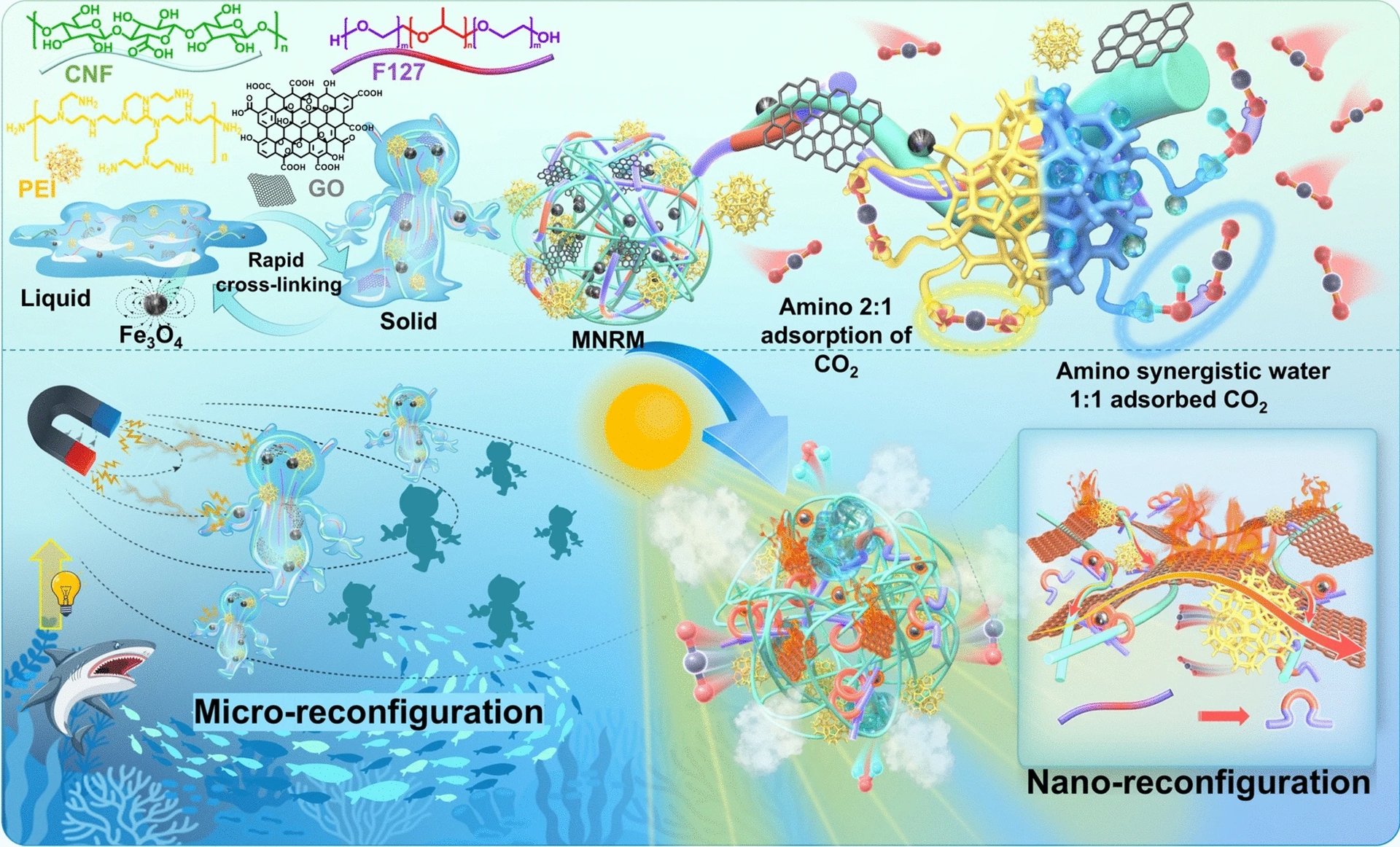
Removing, or “scrubbing”, carbon dioxide from the air of confined spaces is a critical component of any life support system on a spacecraft or submarine. However, modern day ones are energy intensive, requiring temperatures of up to…
Author: admin
-

Golden Globes 2026: Nominations to be announced
Action!published at 11:31 GMT
Image source, Neil Hall/EPA/ShutterstockImage caption, Wicked: For Good has been a fan-favourite at the cinema – but will it win big this awards season?
In case the countdown to Christmas isn’t bringing…
Continue Reading
-

Morning Bid: The final Fed countdown
By Yoruk Bahceli
LONDON, Dec 8 (Reuters) – By Yoruk Bahceli, markets correspondent
What matters in U.S. and global markets today
The Federal Reserve meeting is fast approaching on Wednesday – and the stakes are high. Rising bets for a December cut seem to have saved equities from AI jitters for now. But the effect on the dollar, which dipped further on Monday, has been more pain.
I’ll get into all the market news below.
But first check out Mike Dolan’s latest column where he discusses what is at the heart of the problem facing the Federal Reserve this week and throughout 2026.
And listen to the latest episode of the new Morning Bid daily podcast. Subscribe to hear Mike and other Reuters journalists discuss the biggest news in markets and finance seven days a week.
Today’s Market Minute
* China’s exports topped forecasts in November, driven by asurge in shipments to non-U.S. markets as manufacturers deepentrade ties with the rest of the world in light of PresidentDonald Trump’s prohibitively high tariffs. * A wave of flight cancellations by IndiGo, India’s largestairline, sparked a week of chaos and grounded tens of thousandsof passengers, laying bare the risks of having a duopoly-likesituation in the world’s fastest-growing aviation market. * U.S. lawmakers on Sunday unveiled an annual defense policybill authorizing a record $901 billion in national securityspending next year, billions more than President Donald Trump’srequest, and provides $400 million in military assistance toUkraine. * China’s imports of major commodities were on divergenttracks in November, with crude oil and iron ore powering aheadbut copper and coal losing steam. The contrasting outcomes makeit difficult to come up with a single narrative for demand inthe world’s biggest buyer of natural resources, writes ROICommodities and Energy Columnist Clyde Russell. * The G7’s proposed plan to bar tankers from hauling Russianoil ups the ante in the West’s economic stand-off with Moscow,but the ultimate bite hinges on whether governments will ratchetup punishments on those skirting sanctions, argues ROI EnergyColumnist Ron Bousso
The final Fed countdown
No surprise: It’s all about the Fed this week, with traders pretty confident — and hopeful — the bank will deliver a 25 basis-point rate cut on Wednesday.
After all, bets that the Fed will cut rates have helped the S&P 500 bounce back from AI woes and jump 5% since late November, so equity traders need Jerome Powell and co to deliver.
As for the dollar, the market verdict is more pain.
Continue Reading
-
Know schedule and where to watch live streaming in India
Hosts India will field a four-member squad comprising Anahat Singh, Joshna Chinappa, Abhay Singh and Velavan Senthilkumar at the Squash World Cup 2025, set to kick off in Chennai from Tuesday.
Watch the Squash World Cup 2025 on live streaming in…
Continue Reading
-

Lando Norris inspires children in Somerset to take up motorsport
Clara Bullockand
Joe Sims,Bristol
 PA Media
PA MediaLando Norris became world champion over the weekend Formula 1 World Champion Lando Norris has inspired many young people to take up the sport, his former karting trainer said.
The 26-year-old, who was born in…
Continue Reading
-

PPARγ mediated enhanced lipid biogenesis fuels Mycobacterium tuberculosis growth in a drug-tolerant hepatocyte environment
Over the years, various anatomical and cellular environments conducive to Mtb infection have been identified, particularly concerning its latency (Cosma et al., 2003; Bussi and Gutierrez, 2019). Pulmonary TB typically presents with…
Continue Reading
-

Blyth offshore wind farm marks 25-year milestone
 PA Media
PA MediaThe offshore wind farm in Blyth turns 25 this month The “critical role” played by the UK’s first offshore wind farm has been celebrated 25 years after it was built.
The first turbines were erected at the site in Blyth, Northumberland, in December 2000, with the sector having since become the second largest power source in the country after gas.
Energy minister Michael Shanks marked the milestone with a visit to the site and said offshore wind was the “backbone” of the UK’s clean energy system.
“I don’t think anyone could’ve fully appreciated the critical role that offshore wind would play in the future,” he said.
He added switching on the first turbines at Blyth had marked a “hugely important moment”, putting the UK at the forefront of offshore energy.
According to analysis by energy think tank Ember, there are 47 operational offshore wind farms, supplying nearly a fifth (17%) of Britain’s electricity generation and employing about 40,000 people.
 PA Media
PA MediaThe wind farm is 1km (0.6 miles) off the Northumberland coast Frankie Mayo, an analyst at Ember, said the engineering and innovation in British offshore wind should be “a real point of pride”.
He added: “With seabeds and wind speeds second-to-none for this kind of technology, Britain has truly led the world in showing its potential.”
Labour is pushing ahead with efforts to hit its target to remove almost all fossil fuels from the UK’s electricity supply by 2030.
On whether the government will achieve the goal, Shanks said: “Yes, full stop.”
He added there was momentum in the industry “to build big things and to do it faster”.
The Conservatives have previously said Labour’s “rush” to decarbonise the electricity system would push up electricity prices and cause more hardship for people across Britain.
Continue Reading
-

Assam influencer Dhunu Joni’s clip SPARKS controversy; is it real or fake?
19 minute VIRAL video: Assam influencer Dhunu Joni’s clip SPARKS controversy; is it real or fake?