Justice (retd) Yar Muhammad Khan will take oath as the caretaker chief minister of Gilgit-Baltistan on Tuesday (today), following the end of Chief…
Author: admin
-

How to watch all the ‘Now You See Me’ movies on Prime Video – About Amazon
- How to watch all the ‘Now You See Me’ movies on Prime Video About Amazon
- Now You See Me: Now You Don’t Worldwide Box Office: Crosses $100M Mark During 2nd Weekend But Fails To Break Into 2025’s Top 25 Koimoi
- Now You See Me: Now You…
Continue Reading
-

NASA’s 2025 Astronaut Candidates: Shaping Artemis Exploration
When NASA’s 2025 astronaut candidates arrived at the agency’s Johnson Space Center in Houston this fall, they stepped into history, sharing a common mission to master the skills and teamwork that define NASA’s next era of…
Continue Reading
-
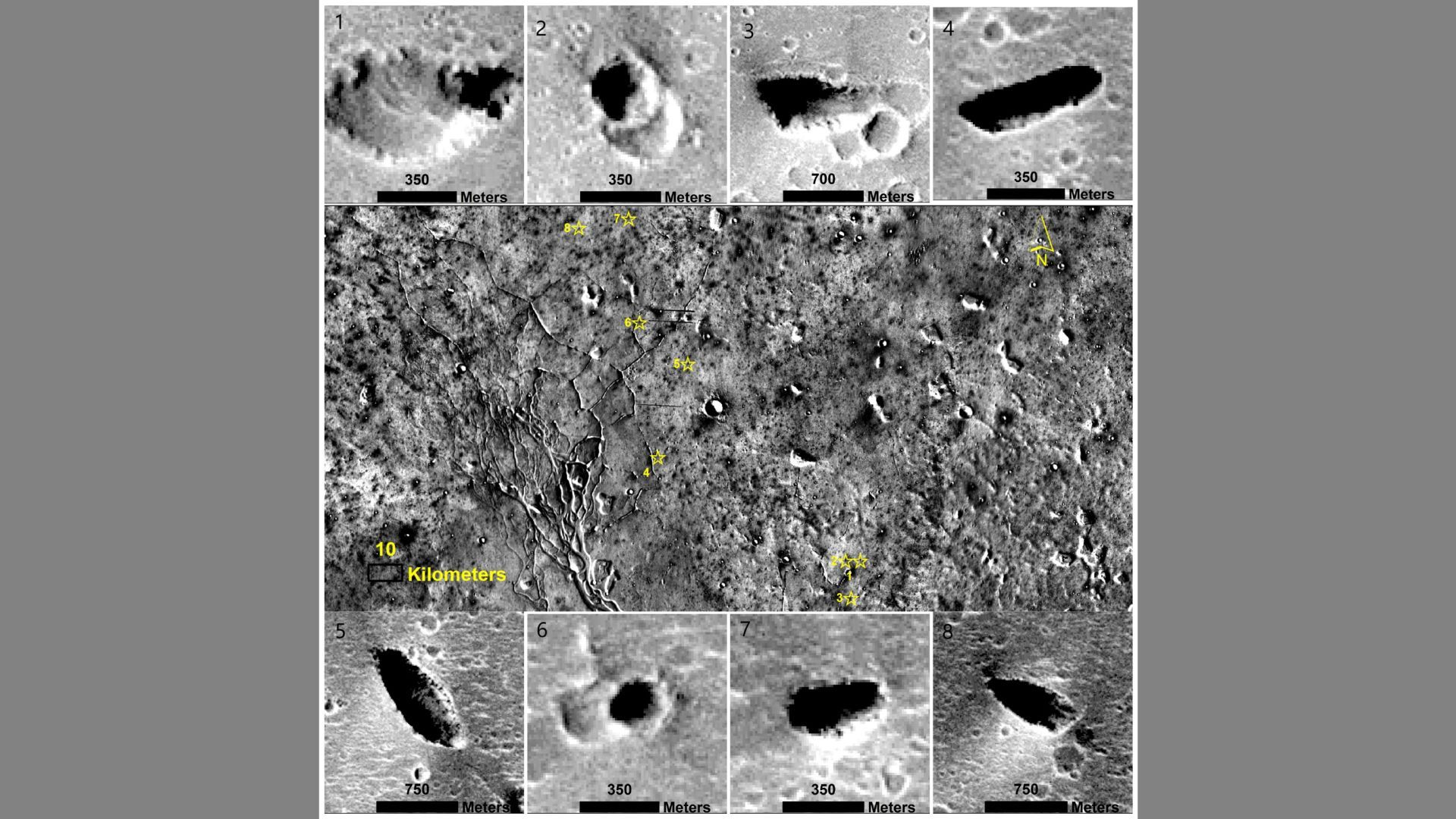
Evidence of ancient life on Mars could be hidden away in colossal water-carved caves
Possible giant “karstic” caves that formed when slightly acidic water dissolved bedrock have been identified on Mars and hailed as one of the best locations on the Red Planet to search for preserved biosignatures.
“With the expected technological…
Continue Reading
-

‘The Morning Show’ Cast and Producers Season 5, Current Events to Tackle
“The Morning Show” has a knack for covering current events — the MeToo movement, space travel and in Season 4, AI. And that reflection of what’s happening in the real world will likely continue into Season 5.
“I think we will be…
Continue Reading

