What Happened: You know that handy “Advanced Paste” trick in Microsoft’s PowerToys? Well, it’s getting a massive brain upgrade that doesn’t need the internet.
- With the new 0.96 update rolling out now, this feature has gone…

What Happened: You know that handy “Advanced Paste” trick in Microsoft’s PowerToys? Well, it’s getting a massive brain upgrade that doesn’t need the internet.

Available for over a year
We celebrate South Africa’s first Test win in India for 15 years, and Charu Sharma tells us what India will look to do differently to ensure they do not lose the two-Test series.
The Ashes is upon us, so Nikesh…

Strictly Come Dancing contestant La Voix is to leave the current competition after sustaining an injury.
The drag queen’s departure was announced by presenter Tess Daly at the start of Saturday’s episode from Blackpool, saying she had had to…

Cardi B has turned a deeply personal moment into a lasting memento following the birth of her son…
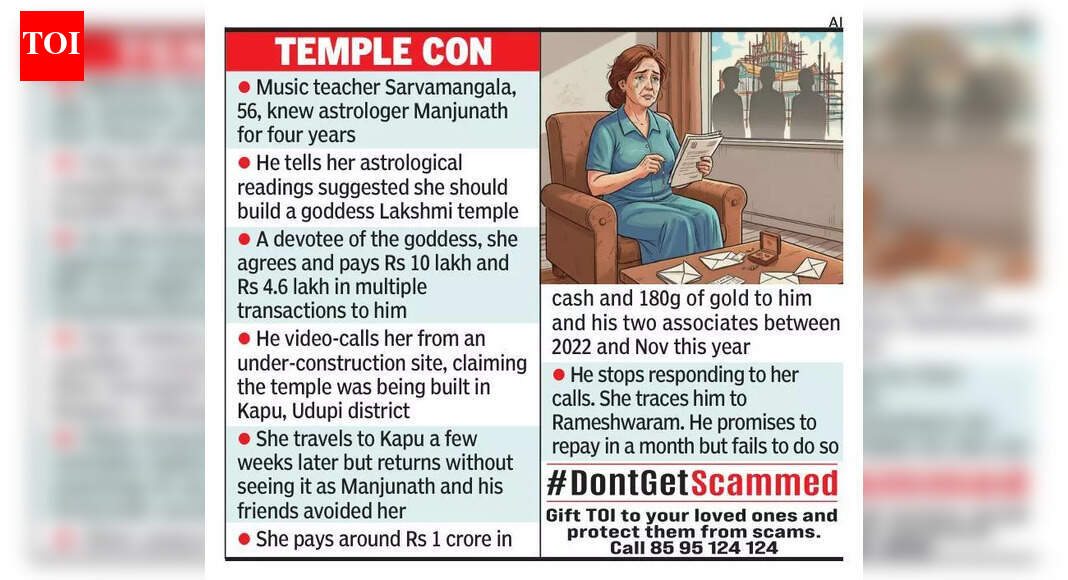
Bengaluru: A 56-year-old music teacher has accused an astrologer and three accomplices of cheating her of nearly Rs 1 crore in cash and 180 grams of gold, allegedly under the pretext of raising funds to build a templeIn her complaint to…


On Nov. 24, CBS will air Everybody Loves Raymond: 30th Anniversary Reunion, a 90-minute special celebrating the Emmy-winning comedy series. While reuniting the surviving cast of the show for the first time is great news for fans, its timing is…

Rescue 1122 teams clear debris from residential houses damaged in a chemical factory explosion in Faisalabad. Photo: INP

Keir Starmer has increased the pressure on Andrew Mountbatten-Windsor to cooperate with a congressional investigation into Jeffrey Epstein, saying those who are caught up in child sexual offence cases should disclose any information they have.
The multicenter, single-arm ReNeu study enrolled patients at least 2 years of age with symptomatic, inoperable NF1-associated PN causing significant morbidity; of the 114 patients enrolled, 56 were children ranging from 2 years of age to 17 years of age, and 58 were adults who were 18 years of age or older.1,3
For the treatment phase, patients were administered mirdametinib as a capsule or dispersible tablet at a twice-daily dose of 2 mg/m2 as part of a 3-weeks-on/1-week-off schedule. Treatment continued until progressive disease or intolerable toxicity.3
After the treatment phase, patients entered a 30-day safety follow-up period, and they were assessed for eligibility for optional LTFU.1 Specifically, 84% of adults (n = 26/31) and 86% of children (n = 32/37) elected to continue mirdametinib in the LTFU period. This was followed by another 30-day safety follow-up period.
Key trial end points comprised BICR-assessed confirmed ORR, which was defined as the percentage of patients who experienced a reduction in target PN volume of at least 20% by MRI on consecutive scans at any time before data cutoff; change in target PN volume from baseline; DOR; and safety and tolerability.
In the adult population, the median age was 34 years (range, 18-69), 64% were female, the median volume of target PN was 196 mL (range, 1-3457), and 53% had target PN progressing at the time of trial entry. The most common location of target PN was head and neck (48%), followed by lower/upper extremities (29%), paraspinal (9%), torso (9%), and other (5%). The most common type of PN-related morbidity was pain (90%), followed by disfigurement or major deformity (52%), motor dysfunction or weakness (40%), other (17%), or airway dysfunction (5%).
In the pediatric population, the median age was 10 years (range, 2-17), 54% were female, the median volume of target PN was 99 mL (range, 5-3630), and 62% of patients had target PN progressing at the time of entering the trial. Again, the most common location of target PN was head and neck (50%), followed by lower/upper extremities (14%), torso (14%), other (14%), and paraspinal (7%). The most common type of PN-related morbidity in this population was again pain (70%), followed by disfigurement or major deformity (50%), motor dysfunction or weakness (27%), other (21%), and airway dysfunction (12%).
No new drug-related safety signals emerged with LTFU.
In the adult population, any-grade treatment-related adverse effects (TRAEs) occurred in 98% of patients, 17% of which were grade 3 or higher. The most common TRAEs experienced by at least 20% of patients included dermatitis acneiform (any grade, 78%; grade ≥3, 9%), diarrhea (48%; 0%), and nausea (36%; 0%). Serious TRAEs were experienced by 2% of patients. Additionally, TRAEs led to dose reductions, interruptions, or discontinuations for 17%, 9%, and 22% of patients, respectively.
In the pediatric population, any-grade TRAEs occurred in 95% of patients; 25% of cases were grade 3 or higher in severity. The most common TRAEs in this population were, again, dermatitis acneiform (any grade, 43%; grade ≥3, 2%), diarrhea (38%; 2%), and nausea (21%; 0%). TRAEs led to dose reductions, interruptions, or discontinuations for 14%, 14%, and 9% of patients, respectively.
“No additional serious TRAEs [and] no additional dose interruptions [were observed]; [there was] 1 additional dose reduction and 1 additional discontinuation due to TRAEs compared to the prior data cutoff,” Hirbe noted in the presentation.
Previous data showed that in adult patients (n = 58), the confirmed ORR was 41% (95% CI, 29%-55%) with 88% of patients experiencing a DOR of at least 12 months and 50% experiencing a DOR of at least 24 months.3 In pediatric patients, the confirmed ORR with mirdametinib was 52% (95% CI, 38%-65%), with 90% and 48% of patients experiencing a DOR lasting for at least 12 or 24 months, respectively. Early, sustained, and clinically meaningful improvements in pain and health-related quality of life (QOL) were also reported.4
The prior data supported the
Moertel serves as a pediatric neuro-oncologist and professor at the University of Minnesota School of Medicine in Minneapolis, where he directs the Pediatric Neuro-Oncology Fellowship Program and co-leads the Pediatric Neuro-Oncology and Neurofibromatosis Programs.
Moreover, in July 2025, the